Record Information Version
1.0 Update Date
1/22/2018 11:54:54 AM
Metabolite ID PAMDB110160
Identification Name:
N -formylkynurenine Description: Not Available
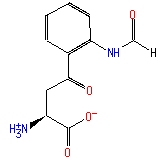
Structure
Synonyms:
Chemical Formula:
C11 H12 N2 O4
Average Molecular Weight:
237.0875319161 Monoisotopic Molecular
Weight:
237.0875319161 InChI Key:
BYHJHXPTQMMKCA-QMMMGPOBSA-N InChI:
InChI=1S/C11H12N2O4/c12-8(11(16)17)5-10(15)7-3-1-2-4-9(7)13-6-14/h1-4,6,8H,5,12H2,(H,13,14)(H,16,17)/t8-/m0/s1 CAS
number:
Not Available IUPAC Name: Not Available
Traditional IUPAC Name:
Not Available SMILES: [CH](=O)NC1(C=CC=CC=1C(=O)CC([N+])C(=O)[O-])
Chemical Taxonomy
Taxonomy Description This compound belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group.
Kingdom
Organic compounds Super Class Organic oxygen compounds
Class
Organooxygen compounds Sub Class Carbonyl compounds
Direct Parent
Alkyl-phenylketones Alternative Parents
Substituents
Alkyl-phenylketone Butyrophenone Alpha-amino acid or derivatives Anilide Benzoyl Gamma-keto acid Aryl alkyl ketone N-arylamide Monocyclic benzene moiety Benzenoid Keto acid Vinylogous amide Carboxamide group Carboxylic acid salt Secondary carboxylic acid amide Carboxylic acid Carboxylic acid derivative Monocarboxylic acid or derivatives Organic zwitterion Organic salt Organic nitrogen compound Hydrocarbon derivative Organic oxide Organonitrogen compound Organopnictogen compound Aromatic homomonocyclic compound Molecular Framework
Aromatic homomonocyclic compounds External Descriptors
Not Available
Physical Properties State:
Not Available Charge: Not Available
Melting point:
Not Available Experimental Properties:
Not Available Predicted Properties
Biological Properties Cellular Locations:
Not Available Reactions:
Pathways:
Spectra Spectra:
Not Available
References References:
Not Available Synthesis Reference:
Not Available Material Safety Data Sheet (MSDS)
Not Available
Links External Links:
This project is supported by
the University of Maryland ,
School of Pharmacy ,
Mass Spectrometry Center , a Waters Center of Excellence. The center is an NIH-investigator funded research and core facility that supports a wide range of cutting-edge metabolomic studies. The Center is supported through a center grant from the University of Maryland and NIH grants to its members. The PAMDB project is affiliated with
The Metabolomics Innovation Centre (TMIC) a leading metabolomics research and service center funded through Genome Alberta, Genome British Columbia and Genome Canada.