|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110158 |
|---|
|
Identification |
|---|
| Name: |
4-imidazolone-5-propanoate |
|---|
| Description: | Conjugate base of 3-(4-oxo-4,5-dihydro-1H-imidazol-5-yl)propanoic acid. |
|---|
|
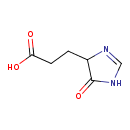
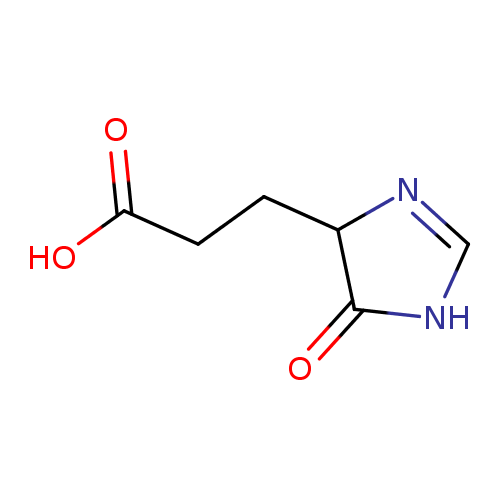
Structure |
|
|---|
| Synonyms: | -
4-imidazolone-5-propionate
-
4-imidazolone-5-propionic acid
-
4,5-dihydro-4-oxo-5-imidazolepropanoate
-
imidazolone propionic acid
-
imidazolonepropanoate
-
imidazolone propionate
-
(S)-3-(5-oxo-4,5-dihydro-3H-imidazol-4-yl)propanoate
-
3-(5-oxo-4,5-dihydro-3H-imidazol-4-yl)propanoate
-
5-(2-carboxylatoethyl)-4-oxo-4,5-dihydro-1H-imidazol-5-ide
|
|---|
|
Chemical Formula: |
C6H7N2O3
|
|---|
| Average Molecular Weight: |
155.13 |
|---|
| Monoisotopic Molecular
Weight: |
156.0534921335 |
|---|
| InChI Key: |
HEXMLHKQVUFYME-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C6H8N2O3/c9-5(10)2-1-4-6(11)8-3-7-4/h3-4H,1-2H2,(H,9,10)(H,7,8,11)/p-1 |
|---|
| CAS
number: |
17340-16-8 |
|---|
| IUPAC Name: | 3-(4-oxo-4,5-dihydro-1H-imidazol-5-id-5-yl)propanoate |
|---|
|
Traditional IUPAC Name: |
3-(5-oxo-1,4-dihydroimidazol-4-yl)propanoic acid |
|---|
| SMILES: | C(C1(C(=O)N=CN1))CC(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Azoles |
|---|
|
Direct Parent |
Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Imidazolyl carboxylic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Niederwieser A, Matasovic A, Steinmann B, Baerlocher K, Kempken B: Hydantoin-5-propionic aciduria in folic acid nondependent formiminoglutamic aciduria observed in two siblings. Pediatr Res. 1976 Apr;10(4):215-9. [1272625 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|