|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110150 |
|---|
|
Identification |
|---|
| Name: |
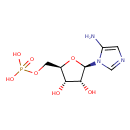
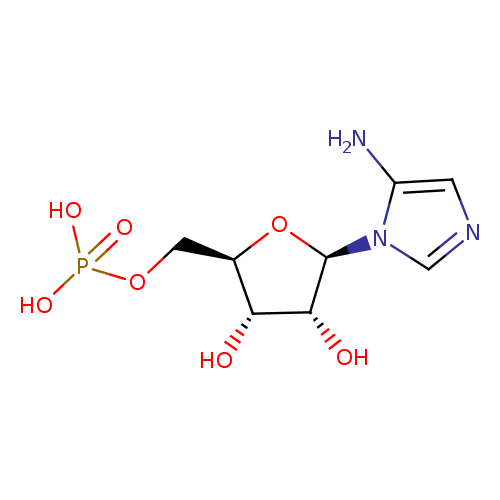
5-amino-1-(5-phospho-β-D-ribosyl)imidazole |
|---|
| Description: | 5-aminoimidazole ribonucleotide (AIR), is an intermediate of purine nucleotide biosynthesis. It is also the precursor to 4-amino-2-methyl-5-hydroxymethylpyrimidine (HMP), the first product of pyrimidine biosynthesis. This reaction is mediated by the enzyme HMP-P kinase (ThiD). HMP is a precursor of thiamine phosphate (TMP), and subsequently to thiamine pyrophosphate (TPP). TPP is an essential cofactor in all living systems that plays a central role in metabolism. (PMID: 15326535 ). 5-Aminoimidazole ribonucleotide is a substrate for a number of proteins including: Scaffold attachment factor B2, Multifunctional protein ADE2, Pulmonary surfactant-associated protein B, Tumor necrosis factor receptor superfamily member 25, Pulmonary surfactant-associated protein C, Serine/threonine-protein kinase Chk1, Vinexin, Trifunctional purine biosynthetic protein adenosine-3, Antileukoproteinase 1 and Scaffold attachment factor B. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
5-aminoimidazole ribonucleotide
-
5-amino-1-(5-phospho-D-ribosyl)imidazole
-
5-aminoimidazole ribotide
-
AIR
-
1-(5'-phosphoribosyl)-5-aminoimidazole
-
5'-phosphoribosyl-5-aminoimidazole
-
phosphoribosylaminoimidazole
|
|---|
|
Chemical Formula: |
C8H13N3O7P
|
|---|
| Average Molecular Weight: |
294.18 |
|---|
| Monoisotopic Molecular
Weight: |
296.0647613618 |
|---|
| InChI Key: |
PDACUKOKVHBVHJ-XVFCMESISA-M |
|---|
| InChI: |
InChI=1S/C8H14N3O7P/c9-5-1-10-3-11(5)8-7(13)6(12)4(18-8)2-17-19(14,15)16/h1,3-4,6-8,12-13H,2,9H2,(H2,14,15,16)/p-1/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
25635-88-5 |
|---|
| IUPAC Name: | 1-(5-O-phosphono-D-ribofuranosyl)-1H-imidazol-5-amine |
|---|
|
Traditional IUPAC Name: |
5-aminoimidazole ribotide |
|---|
| SMILES: | C2([N+]=CN(C1(OC(COP([O-])(=O)[O-])C(O)C(O)1))C(N)=2) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Pentose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose phosphate
- Pentose-5-phosphate
- Imidazole ribonucleoside
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Aminoimidazole
- Alkyl phosphate
- Primary aromatic amine
- Phosphoric acid ester
- N-substituted imidazole
- Organic phosphoric acid derivative
- Heteroaromatic compound
- Azole
- Imidazole
- Oxolane
- 1,2-diol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Organic oxide
- Primary amine
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Amine
- Organopnictogen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Vetvik H, Grewal HM, Haugen IL, Ahren C, Haneberg B: Mucosal antibodies can be measured in air-dried samples of saliva and feces. J Immunol Methods. 1998 Jun 1;215(1-2):163-72. [9744758 ]
- Ogata M, Michitsuji H, Fujiki Y: Estimating amounts of toluene inhaled by workers with protective mask using biological indicators of toluene. Toxicol Lett. 1999 Sep 5;108(2-3):233-9. [10511267 ]
- Chang HK, Weber ME, King M: Mucus transport by high-frequency nonsymmetrical oscillatory airflow. J Appl Physiol. 1988 Sep;65(3):1203-9. [3182490 ]
- Lawhorn BG, Mehl RA, Begley TP: Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Org Biomol Chem. 2004 Sep 7;2(17):2538-46. Epub 2004 Aug 11. [15326535 ]
|
|---|
| Synthesis Reference: |
Groziak M P; Bhat B; Leonard N J Nonenzymatic synthesis of 5-aminoimidazole ribonucleoside and recognition of its facile rearrangement. Proceedings of the National Academy of Sciences of the United States of America (1988), 85(19), 7174-6. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|