|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110139 |
|---|
|
Identification |
|---|
| Name: |
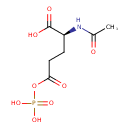
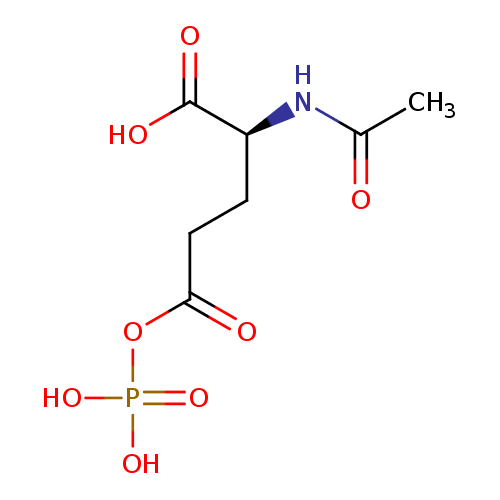
N-acetylglutamyl-phosphate |
|---|
| Description: | Trianion of N-acetyl-L-γ-glutamyl phosphate arising from deprotonation of carboxy and phosphate groups; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
N-Acetyl-L-glutamyl 5-phosphate
-
N-acetyl-L-glutamate-5-phosphate
-
N-acetyl-glutamyl-P
-
N-acetylglutamyl-P
-
N-acetyl-5-glutamyl phosphate
|
|---|
|
Chemical Formula: |
C7H9NO8P
|
|---|
| Average Molecular Weight: |
266.12 |
|---|
| Monoisotopic Molecular
Weight: |
269.0300528772 |
|---|
| InChI Key: |
FCVIHFVSXHOPSW-YFKPBYRVSA-K |
|---|
| InChI: |
InChI=1S/C7H12NO8P/c1-4(9)8-5(7(11)12)2-3-6(10)16-17(13,14)15/h5H,2-3H2,1H3,(H,8,9)(H,11,12)(H2,13,14,15)/p-3/t5-/m0/s1 |
|---|
| CAS
number: |
15383-57-0 |
|---|
| IUPAC Name: | (2S)-2-acetamido-5-oxo-5-(phosphonatooxy)pentanoate |
|---|
|
Traditional IUPAC Name: |
(2S)-2-acetamido-5-oxo-5-(phosphonooxy)pentanoic acid |
|---|
| SMILES: | CC(=O)NC(C([O-])=O)CCC(=O)OP([O-])(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glutamic acid and derivatives. These are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Glutamic acid and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glutamic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Acyl monophosphate
- Acyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Fatty acid
- Acetamide
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Kuramitsu, Shigenori; Masui, Ryoji. Cloning of thermostable acetylglutamate kinase gene from Sulfolobus tokodaii and use for N-acetyl-L-glutamate-5-phosphate biosynthesis. Jpn. Kokai Tokkyo Koho (2004), 12 pp. CODEN: JKXXAF JP 2004298187 A 20041028 CAN 141:362386 AN 2004:900796 |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|