|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110127 |
|---|
|
Identification |
|---|
| Name: |
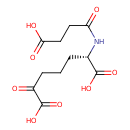
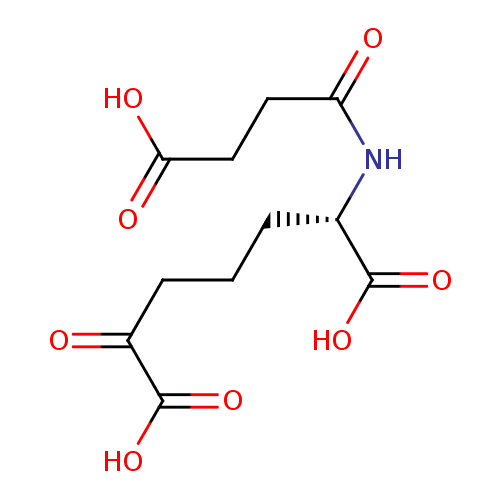
N-succinyl-2-amino-6-ketopimelate |
|---|
| Description: | N-Succinyl-2-amino-6-ketopimelate is an intermediate in lysine biosynthesis. It is the fourth to last step in the synthesis of lysine and is converted. from tetrahydrodipicolinate via the enzyme tetrahydrodipicolinate N-succinyltransferase (EC 2.3.1.117). It is then converted to N-succinyl-L,L-2,6-diaminopimelate via the enzyme Succinyldiaminopimelate transferase (EC 2.6.1.17). |
|---|
|
Structure |
|
|---|
| Synonyms: | -
N-Succinyl-2-L-amino-6-oxoheptanedioate
-
N-succinyl-L-2-amino-6-oxoheptanedioate
-
succinyl-ε-keto-α-aminopimelate
|
|---|
|
Chemical Formula: |
C11H12NO8
|
|---|
| Average Molecular Weight: |
286.22 |
|---|
| Monoisotopic Molecular
Weight: |
289.0797664635 |
|---|
| InChI Key: |
SDVXSCSNVVZWDD-LURJTMIESA-K |
|---|
| InChI: |
InChI=1S/C11H15NO8/c13-7(11(19)20)3-1-2-6(10(17)18)12-8(14)4-5-9(15)16/h6H,1-5H2,(H,12,14)(H,15,16)(H,17,18)(H,19,20)/p-3/t6-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2S)-2-(3-carboxylatopropanamido)-6-oxoheptanedioate |
|---|
|
Traditional IUPAC Name: |
(2S)-2-(3-carboxypropanamido)-6-oxoheptanedioic acid |
|---|
| SMILES: | C(CC(=O)C(=O)[O-])CC(NC(CCC([O-])=O)=O)C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as n-acyl-l-alpha-amino acids. These are n-acylated alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
N-acyl-L-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-l-alpha-amino acid
- Tricarboxylic acid or derivatives
- Alpha-keto acid
- Fatty amide
- Keto acid
- Fatty acyl
- N-acyl-amine
- Alpha-hydroxy ketone
- Carboxamide group
- Secondary carboxylic acid amide
- Ketone
- Carboxylic acid
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- superpathway of L-lysine, L-threonine and L-methionine biosynthesis IP4-PWY
- L-lysine biosynthesis IDAPLYSINESYN-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|