|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110109 |
|---|
|
Identification |
|---|
| Name: |
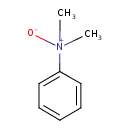
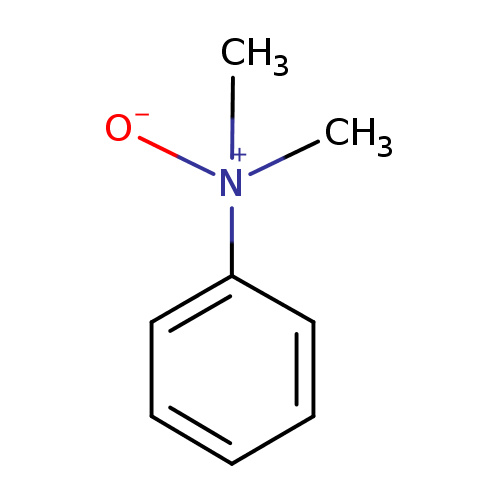
N,N-dimethylaniline-N-oxide |
|---|
| Description: | Dimethylaniline-N-oxide is a substrate for Dimethylaniline monooxygenase 4, Dimethylaniline monooxygenase 3, Dimethylaniline monooxygenase 1, Dimethylaniline monooxygenase 5, Putative dimethylaniline monooxygenase 6 and Dimethylaniline monooxygenase 2. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Dimethyl(phenyl)amine oxide
- Dimethylaniline N-oxide
- N,N-Dimethylaniline N-oxide
- NN-Dimethylaniline-N-oxide
- Dimethylaniline N-oxide hydrochloride
|
|---|
|
Chemical Formula: |
C8H11NO
|
|---|
| Average Molecular Weight: |
137.18 |
|---|
| Monoisotopic Molecular
Weight: |
137.0840639804 |
|---|
| InChI Key: |
LKQUDAOAMBKKQW-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C8H11NO/c1-9(2,10)8-6-4-3-5-7-8/h3-7H,1-2H3 |
|---|
| CAS
number: |
874-52-2 |
|---|
| IUPAC Name: | N,N-dimethylaniline N-oxide |
|---|
|
Traditional IUPAC Name: |
dimethylaniline N-oxide |
|---|
| SMILES: | CN(C)(=O)C1(C=CC=CC=1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
Benzene and substituted derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monocyclic benzene moiety
- Trisubstituted n-oxide
- N-oxide
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Ziegler, Daniel M.; Pettit, Flora H. Formation of an intermediate N-oxide in the oxidative demethylation of N,N-dimethylaniline catalyzed by liver microsomes. Biochemical and Biophysical Research Communications (1964), 15(2), 188-93. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|