|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110107 |
|---|
|
Identification |
|---|
| Name: |
adenosine 5'-phosphosulfate |
|---|
| Description: | An organic dianion arising from deprotonation of the phosphate and sufate groups of 5'-adenylyl sulfate; major species at pH 7.3. |
|---|
|
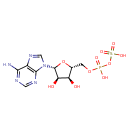
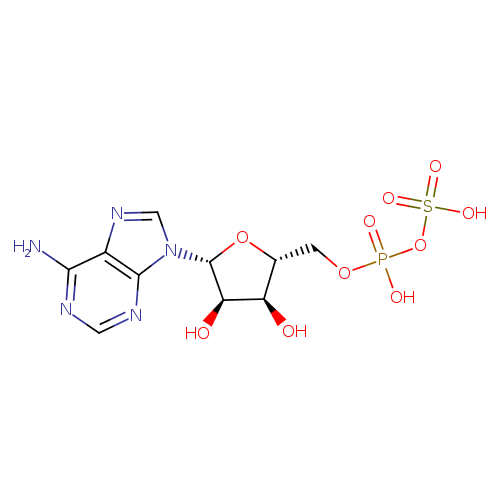
Structure |
|
|---|
| Synonyms: | -
adenylyl-sulfate
-
APS
-
adenosine phosphosulfate
-
adenosine 5'-sulphatophosphate
|
|---|
|
Chemical Formula: |
C10H12N5O10PS
|
|---|
| Average Molecular Weight: |
425.27 |
|---|
| Monoisotopic Molecular
Weight: |
427.0198988964 |
|---|
| InChI Key: |
IRLPACMLTUPBCL-KQYNXXCUSA-L |
|---|
| InChI: |
InChI=1S/C10H14N5O10PS/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-26(18,19)25-27(20,21)22/h2-4,6-7,10,16-17H,1H2,(H,18,19)(H2,11,12,13)(H,20,21,22)/p-2/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
485-84-7 |
|---|
| IUPAC Name: | 5'-O-[(sulfonatooxy)phosphinato]adenosine |
|---|
|
Traditional IUPAC Name: |
adenosine phosphosulfate |
|---|
| SMILES: | C(C3(C(C(C(N2(C1(=C(C(=NC=N1)N)N=C2)))O3)O)O))OP(OS(=O)([O-])=O)([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Alkyl phosphate
- Imidolactam
- Pyrimidine
- Monosaccharide
- Primary aromatic amine
- N-substituted imidazole
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Azole
- Imidazole
- Organic sulfuric acid or derivatives
- Oxolane
- Heteroaromatic compound
- 1,2-diol
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Alcohol
- Primary amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Raju U, Kadner S, Levitz M, Kaganowicz A, Blaustein A: Glucosiduronidation and esterification of androsterone by human breast tumors in vitro. Steroids. 1981 Apr;37(4):399-407. [7245287 ]
- Mateos-Trigos G, Evans RJ, Heath MF: Effects of P2Y(1) and P2Y(12) receptor antagonists on ADP-induced shape change of equine platelets: comparison with human platelets. Platelets. 2002 Aug-Sep;13(5-6):285-92. [12189014 ]
- Chou HC, Lang NP, Kadlubar FF: Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s). Cancer Res. 1995 Feb 1;55(3):525-9. [7834621 ]
- Al-Buheissi SZ, Patel HR, Meinl W, Hewer A, Bryan RL, Glatt H, Miller RA, Phillips DH: N-Acetyltransferase and sulfotransferase activity in human prostate: potential for carcinogen activation. Pharmacogenet Genomics. 2006 Jun;16(6):391-9. [16708048 ]
- Keogh JR, Wolf MF, Overend ME, Tang L, Eaton JW: Biocompatibility of sulphonated polyurethane surfaces. Biomaterials. 1996 Oct;17(20):1987-94. [8894093 ]
- Eto Y, Tokoro T, Handa T, Herschkowitz NN, Rennert OM: Acid mucopolysaccharide (AMPS) abnormality in multiple sulfatase deficiency: chemical compositions of AMPS in urine and liver. Pediatr Res. 1982 May;16(5):395-9. [6212904 ]
- Slomiany BL, Liau YH, Sarosiek J, Tsukada H, Mizuta K, Rosenthal W, Slomiany A: Sulfation of glycolipids by human gastric mucosa in disease. Digestion. 1987;36(4):246-52. [3475228 ]
- Eklund E, Roden L, Malmstrom M, Malmstrom A: Dermatan is a better substrate for 4-O-sulfation than chondroitin: implications in the generation of 4-O-sulfated, L-iduronate-rich galactosaminoglycans. Arch Biochem Biophys. 2000 Nov 15;383(2):171-7. [11185550 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|