|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110106 |
|---|
|
Identification |
|---|
| Name: |
hydroxypyruvate |
|---|
| Description: | A hydroxy monocarboxylic acid anion that results from the deprotonation of the carboxylic acid group of 3-hydroxypyruvic acid. |
|---|
|
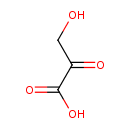
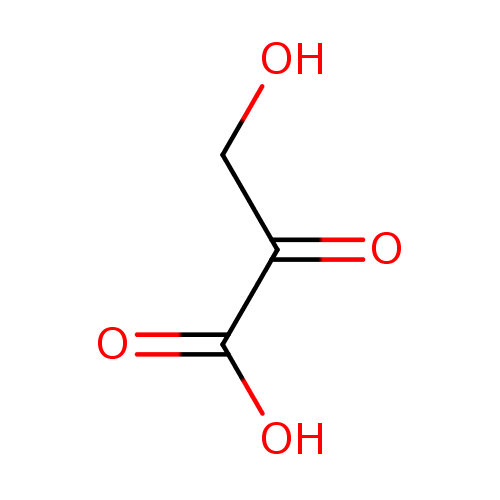
Structure |
|
|---|
| Synonyms: | -
β-hydroxypyruvate
-
OH-pyruvate
-
OH-pyr
-
3-hydroxypyruvate
|
|---|
|
Chemical Formula: |
C3H3O4
|
|---|
| Average Molecular Weight: |
103.05 |
|---|
| Monoisotopic Molecular
Weight: |
104.0109586168 |
|---|
| InChI Key: |
HHDDCCUIIUWNGJ-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C3H4O4/c4-1-2(5)3(6)7/h4H,1H2,(H,6,7)/p-1 |
|---|
| CAS
number: |
1113-60-6 |
|---|
| IUPAC Name: | 3-hydroxy-2-oxopropanoate |
|---|
|
Traditional IUPAC Name: |
hydroxypyruvic acid |
|---|
| SMILES: | C(C(=O)C([O-])=O)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as beta hydroxy acids and derivatives. These are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Hydroxy acids and derivatives |
|---|
|
Direct Parent |
Beta hydroxy acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Beta-hydroxy acid
- Alpha-keto acid
- Keto acid
- Monosaccharide
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kolvraa S, Rasmussen K, Brandt NJ: D-glyceric acidemia: biohcemical studies of a new syndrome. Pediatr Res. 1976 Oct;10(10):825-30. [972784 ]
|
|---|
| Synthesis Reference: |
Behal, Francis J. Hydroxypyruvic acid formation in Aspergillus niger. Archives of Biochemistry and Biophysics (1960), 88 110-12. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|