|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110105 |
|---|
|
Identification |
|---|
| Name: |
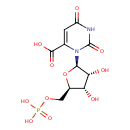
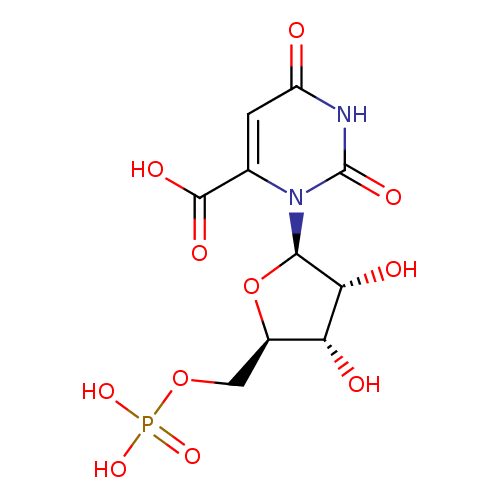
orotidine 5'-phosphate |
|---|
| Description: | Trianion of orotidine 5'-phosphate arising from deprotonation of carboxylic acid and phosphate functions. |
|---|
|
Structure |
|
|---|
| Synonyms: | - uridine-5\'-phosphate biosynthesis
- de novo biosynthesis of uridine-5\'-phosphate
de novo biosynthesis of uridine-5\'-monophosphate'- WIDTH
- 500);" onmouseout="return nd();"> UMP biosynthesis
:
orotidine 5'-phosphate + H+ → CO2 + UMP
|
|---|
|
Chemical Formula: |
C10H10N2O11P
|
|---|
| Average Molecular Weight: |
365.17 |
|---|
| Monoisotopic Molecular
Weight: |
368.0256957808 |
|---|
| InChI Key: |
KYOBSHFOBAOFBF-XVFCMESISA-K |
|---|
| InChI: |
InChI=1S/C10H13N2O11P/c13-5-1-3(9(16)17)12(10(18)11-5)8-7(15)6(14)4(23-8)2-22-24(19,20)21/h1,4,6-8,14-15H,2H2,(H,16,17)(H,11,13,18)(H2,19,20,21)/p-3/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
2149-82-8 |
|---|
| IUPAC Name: | 2,6- dioxo- dioxo- 3- 3- (5- (5- O- O- phosphonato- phosphonato- β- β- D- D- ribofuranosyl)- ribofuranosyl)- 1,2,3,6- 1,2,3,6- tetrahydropyrimidine- tetrahydropyrimidine- 4- 4- carboxylate carboxylate |
|---|
|
Traditional IUPAC Name: |
6-carboxy-5'-uridylic acid |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N2(C(C(=O)[O-])=CC(=O)NC(=O)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine ribonucleoside monophosphates. These are pyrimidine ribobucleotides with monophosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine ribonucleoside monophosphate
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Pyrimidine-6-carboxylic acid or derivatives
- Pyrimidine-6-carboxylic acid
- Hydropyrimidine carboxylic acid derivative
- Monoalkyl phosphate
- Pyrimidone
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Pyrimidine
- Heteroaromatic compound
- Oxolane
- Vinylogous amide
- Secondary alcohol
- Urea
- Lactam
- 1,2-diol
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Oxacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- pyrimidine ribonucleoside 5'-monophosphate (CHEBI:15842 )
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Traut TW: Uridine-5'-phosphate synthase: evidence for substrate cycling involving this bifunctional protein. Arch Biochem Biophys. 1989 Jan;268(1):108-15. [2912371 ]
|
|---|
| Synthesis Reference: |
Ueda, Tohru; Yamamoto, Miyako; Yamane, Akira; Imazawa, Masaoki; Inoue, Hideo. Nucleosides and nucleotides. XXIII. Conversion of uridine nucleotides to the 6-cyano derivatives: synthesis of orotidylic acid. Journal of Carbohydrates, Nucleosides, Nucleotides (1978), 5(3), 261-71. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|