|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110073 |
|---|
|
Identification |
|---|
| Name: |
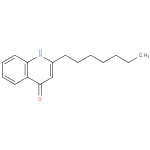
2-heptyl-4(1H)-quinolone |
|---|
| Description: | A quinolone consisting of quinolin-4(1H)-one carrying a heptyl substituent at position 2. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C16H21NO
|
|---|
| Average Molecular Weight: |
243.1623143014 |
|---|
| Monoisotopic Molecular
Weight: |
243.1623143014 |
|---|
| InChI Key: |
UYRHHBXYXSYGHA-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C16H21NO/c1-2-3-4-5-6-9-13-12-16(18)14-10-7-8-11-15(14)17-13/h7-8,10-12H,2-6,9H2,1H3,(H,17,18) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-heptylquinolin-4(1H)-one |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CCCCCCCC1(=CC(=O)C2(=C(N1)C=CC=C2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroquinolones. These are compounds containing a hydrogenated quinoline bearing a ketone group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Quinolines and derivatives |
|---|
| Sub Class | Quinolones and derivatives |
|---|
|
Direct Parent |
Hydroquinolones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Dihydroquinolone
- Dihydroquinoline
- Benzenoid
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- quinolone (CHEBI:62219)
- a tautomer (CPD-12836)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- 2-heptyl-3-hydroxy-4(1H)-quinolone biosynthesisPWY-6660
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Ha DG, Merritt JH, Hampton TH, Hodgkinson JT, Janecek M, Spring DR, Welch M, O'Toole GA (2011)2-Heptyl-4-quinolone, a precursor of the Pseudomonas quinolone signal molecule, modulates swarming motility in Pseudomonas aeruginosa. Journal of bacteriology 193, Pubmed: 21965567
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|