|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110065 |
|---|
|
Identification |
|---|
| Name: |
4-guanidinobutanoate |
|---|
| Description: | Zwitterionic form of 4-guanidinobutanoic acid. |
|---|
|
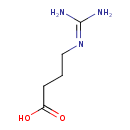
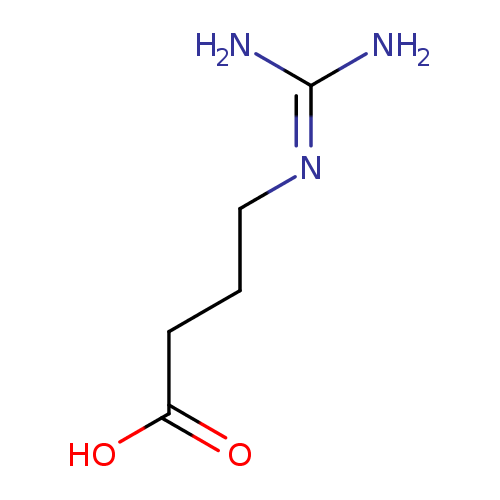
Structure |
|
|---|
| Synonyms: | -
4-guanido-butyrate
-
γ-guanidinobutyrate
-
4-guanidinobutyrate
|
|---|
|
Chemical Formula: |
C5H11N3O2
|
|---|
| Average Molecular Weight: |
146.092951645 |
|---|
| Monoisotopic Molecular
Weight: |
146.092951645 |
|---|
| InChI Key: |
TUHVEAJXIMEOSA-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C5H11N3O2/c6-5(7)8-3-1-2-4(9)10/h1-3H2,(H,9,10)(H4,6,7,8) |
|---|
| CAS
number: |
463-00-3 |
|---|
| IUPAC Name: | 4-{[amino(iminio)methyl]amino}butanoate |
|---|
|
Traditional IUPAC Name: |
4-[(diaminomethylidene)amino]butanoic acid |
|---|
| SMILES: | C([O-])(=O)CCCNC(=[N+])N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as gamma amino acids and derivatives. These are amino acids having a (-NH2) group attached to the gamma carbon atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Gamma amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Gamma amino acid or derivatives
- Straight chain fatty acid
- Fatty acid
- Fatty acyl
- Guanidine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-2900000000-fde97cd959d0a824fb1a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9000000000-7d6981f3d74ac35fbae3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-052r-9000000000-566db10c9a42f3463f73 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0006-0900000000-9133b9248cec58ba4611 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0udi-0900000000-839146a1223d81fcfe1e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0ue9-7900000000-e5db002aeb9f842883d4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-000x-9000000000-272ecb5c7a854deaabb6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-dcdfb92af31da28b06e6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0002-0900000000-57afcc249d5db66b3faf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-000j-9700000000-20d2b0d6d24335aaf532 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-000i-9000000000-a4bb465c44540f709872 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-00kr-9000000000-2803a79b84b073387a24 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0007-9000000000-05ad8804b2be6f14b017 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Mizutani N, Hayakawa C, Ohya Y, Watanabe K, Watanabe Y, Mori A: Guanidino compounds in hyperargininemia. Tohoku J Exp Med. 1987 Nov;153(3):197-205. [3433275 ]
- Marescau B, Qureshi IA, De Deyn P, Letarte J, Ryba R, Lowenthal A: Guanidino compounds in plasma, urine and cerebrospinal fluid of hyperargininemic patients during therapy. Clin Chim Acta. 1985 Feb 28;146(1):21-7. [3987036 ]
- Gatti R, Gioia MG: Anisoin: a useful pre-chromatographic derivatization fluorogenic reagent for LC analysis of guanidino compounds. J Pharm Biomed Anal. 2006 Sep 11;42(1):11-6. Epub 2006 Feb 3. [16460903 ]
- De Deyn PP, Marescau B, Cuykens JJ, Van Gorp L, Lowenthal A, De Potter WP: Guanidino compounds in serum and cerebrospinal fluid of non-dialyzed patients with renal insufficiency. Clin Chim Acta. 1987 Jul 30;167(1):81-8. [3665089 ]
- Marescau B, De Deyn PP, Holvoet J, Possemiers I, Nagels G, Saxena V, Mahler C: Guanidino compounds in serum and urine of cirrhotic patients. Metabolism. 1995 May;44(5):584-8. [7752905 ]
|
|---|
| Synthesis Reference: |
Pisano, John J.; Mitoma, Chozo; Udenfriend, Sidney. Biosynthesis of g-guanidinobutyric acid from g-aminobutyric acid and arginine. Nature (London, United Kingdom) (1957), 180 1125-6. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|