|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110047 |
|---|
|
Identification |
|---|
| Name: |
histidinal |
|---|
| Description: | An organic cation that is the conjugate acid of L-histidinal, arising from protonation of the amino group; major species at pH 7.3. |
|---|
|
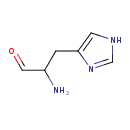
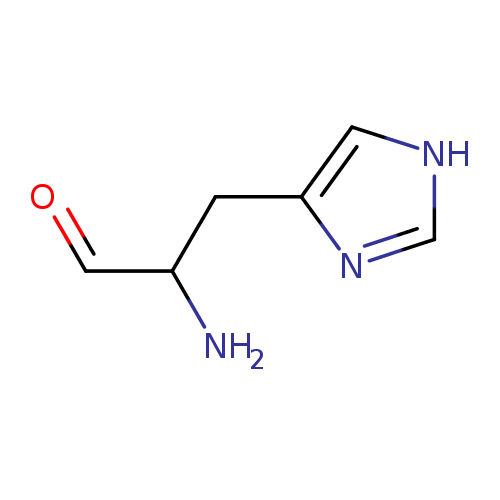
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C6H10N3O
|
|---|
| Average Molecular Weight: |
140.16 |
|---|
| Monoisotopic Molecular
Weight: |
140.0823869587 |
|---|
| InChI Key: |
VYOIELONWKIZJS-YFKPBYRVSA-O |
|---|
| InChI: |
InChI=1S/C6H9N3O/c7-5(3-10)1-6-2-8-4-9-6/h2-5H,1,7H2,(H,8,9)/p+1/t5-/m0/s1 |
|---|
| CAS
number: |
23784-15-8 |
|---|
| IUPAC Name: | (2S)-1-(1H-imidazol-4-yl)-3-oxopropan-2-aminium |
|---|
|
Traditional IUPAC Name: |
2-amino-3-(1H-imidazol-4-yl)propanal |
|---|
| SMILES: | C1(NC=NC=1CC([CH]=O)[N+]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic nitrogen compounds |
|---|
| Sub Class | Organonitrogen compounds |
|---|
|
Direct Parent |
Aralkylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Aralkylamine
- Azole
- Imidazole
- Heteroaromatic compound
- Organoheterocyclic compound
- Azacycle
- Organic oxide
- Aldehyde
- Primary amine
- Organooxygen compound
- Primary aliphatic amine
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | +1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- superpathway of histidine, purine, and pyrimidine biosynthesisPRPP-PWY
- L-histidine biosynthesisHISTSYN-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|