|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110032 |
|---|
|
Identification |
|---|
| Name: |
imidazole acetol-phosphate |
|---|
| Description: | Dianion of 3-(imidazol-4-yl)-2-oxopropyl dihydrogen phosphate arising from deprotonation of both phosphate OH groups; major species at pH 7.3. |
|---|
|
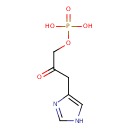
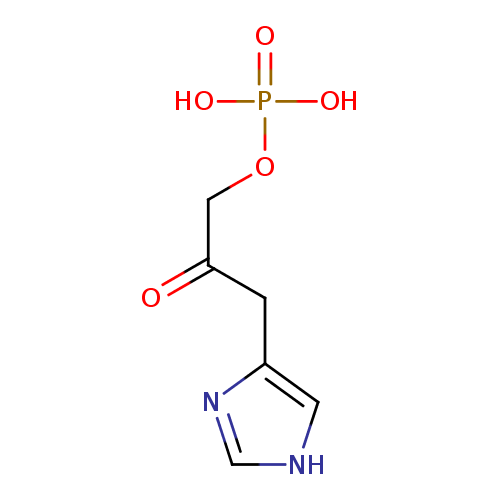
Structure |
|
|---|
| Synonyms: | -
imidazole acetol-P
-
3-(imidazol-4-yl)-2-oxopropyl phosphate
|
|---|
|
Chemical Formula: |
C6H7N2O5P
|
|---|
| Average Molecular Weight: |
218.1 |
|---|
| Monoisotopic Molecular
Weight: |
220.0249079198 |
|---|
| InChI Key: |
YCFFMSOLUMRAMD-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/C6H9N2O5P/c9-6(3-13-14(10,11)12)1-5-2-7-4-8-5/h2,4H,1,3H2,(H,7,8)(H2,10,11,12)/p-2 |
|---|
| CAS
number: |
99979-59-6 |
|---|
| IUPAC Name: | 3-(1H-imidazol-4-yl)-2-oxopropyl phosphate |
|---|
|
Traditional IUPAC Name: |
3-(1H-imidazol-4-yl)-2-oxopropoxyphosphonic acid |
|---|
| SMILES: | C1(NC=NC=1CC(COP([O-])(=O)[O-])=O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Organic phosphoric acids and derivatives |
|---|
|
Direct Parent |
Monoalkyl phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monoalkyl phosphate
- Azole
- Imidazole
- Heteroaromatic compound
- Ketone
- Organoheterocyclic compound
- Azacycle
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- superpathway of histidine, purine, and pyrimidine biosynthesisPRPP-PWY
- L-histidine biosynthesisHISTSYN-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- AMES BN, HORECKER BL: The biosynthesis of histidine: imidazoleacetol phosphate transaminase. J Biol Chem. 1956 May;220(1):113-28. [13319331 ]
- Albritton WL, Levin AP: Some comparative kinetic data on the enzyme imidazoleacetol phosphate:L-glutamate aminotransferase derived from mutant strains of Salmonella typhimurium. J Biol Chem. 1970 May 25;245(10):2525-8. [5445798 ]
- AMES BN, MITCHELL HK: The biosynthesis of histidine; imidazoleglycerol phosphate, imidazoleacetol phosphate, and histidinol phosphate. J Biol Chem. 1955 Feb;212(2):687-96. [14353870 ]
- LEVIN AP, HARTMAN PE: ACTION OF A HISTIDINE ANALOGUE, 1,2,4-TRIAZOLE-3-ALANINE, IN SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Oct;86:820-8. [14066480 ]
- Henderson GB, Snell EE: Vitamin B 6 -responsive histidine deficiency in mutants of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2903-7. [4943547 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|