|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110003 |
|---|
|
Identification |
|---|
| Name: |
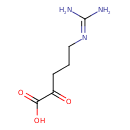
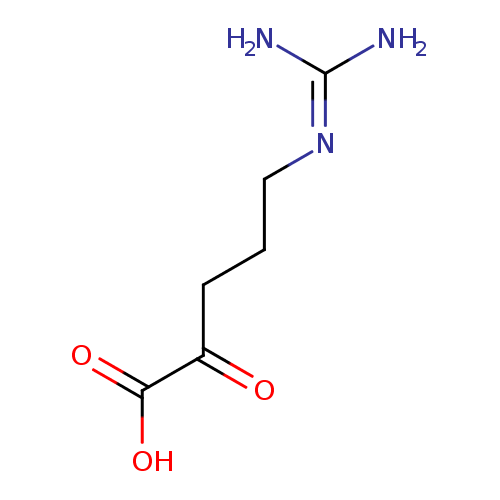
5-guanidino-2-oxo-pentanoate |
|---|
| Description: | Zwitterionic form of 5-guanidino-2-oxopentanoic acid. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
2-oxo-5-guanidinopentanoate
-
2-oxo-5-guanidino-pentanoate
-
5-carbamimidamido-2-oxopentanoate
-
α-ketoarginine
-
2-oxoarginine
-
5-guanidino-2-oxopentanoate
-
2-keto-5-guanidinovalerate
-
2-ketoarginine
|
|---|
|
Chemical Formula: |
C6H11N3O3
|
|---|
| Average Molecular Weight: |
173.17 |
|---|
| Monoisotopic Molecular
Weight: |
174.0878662671 |
|---|
| InChI Key: |
ARBHXJXXVVHMET-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C6H11N3O3/c7-6(8)9-3-1-2-4(10)5(11)12/h1-3H2,(H,11,12)(H4,7,8,9) |
|---|
| CAS
number: |
3715-10-4 |
|---|
| IUPAC Name: | 5-{[amino(iminio)methyl]amino}-2-oxopentanoate |
|---|
|
Traditional IUPAC Name: |
5-[(diaminomethylidene)amino]-2-oxopentanoic acid |
|---|
| SMILES: | C(C(CCCNC(N)=[N+])=O)(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Keto acids and derivatives |
|---|
|
Direct Parent |
Short-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Short-chain keto acid
- Alpha-keto acid
- Alpha-hydroxy ketone
- Guanidine
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- D-Arginine and D-Ornithine Metabolism pae00472
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Mizutani N, Hayakawa C, Ohya Y, Watanabe K, Watanabe Y, Mori A: Guanidino compounds in hyperargininemia. Tohoku J Exp Med. 1987 Nov;153(3):197-205. [3433275 ]
- Marescau B, Qureshi IA, De Deyn P, Letarte J, Ryba R, Lowenthal A: Guanidino compounds in plasma, urine and cerebrospinal fluid of hyperargininemic patients during therapy. Clin Chim Acta. 1985 Feb 28;146(1):21-7. [3987036 ]
- Marescau B, De Deyn PP, Lowenthal A, Qureshi IA, Antonozzi I, Bachmann C, Cederbaum SD, Cerone R, Chamoles N, Colombo JP, et al.: Guanidino compound analysis as a complementary diagnostic parameter for hyperargininemia: follow-up of guanidino compound levels during therapy. Pediatr Res. 1990 Mar;27(3):297-303. [1690873 ]
- Marescau B, Deshmukh DR, Kockx M, Possemiers I, Qureshi IA, Wiechert P, De Deyn PP: Guanidino compounds in serum, urine, liver, kidney, and brain of man and some ureotelic animals. Metabolism. 1992 May;41(5):526-32. [1588833 ]
- Wiechert P, Mortelmans J, Lavinha F, Clara R, Terheggen HG, Lowenthal A: Excretion of guanidino-derivates in urine of hyperargininemic patients. J Genet Hum. 1976 Mar;24(1):61-72. [819629 ]
|
|---|
| Synthesis Reference: |
Chibata, Ichiro; Kakimoto, Toshio; Nabe, Koichi; Shibatani, Takeji. a-Keto-d-guanidinovalerianic acid. Jpn. Kokai Tokkyo Koho (1975), 4 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|