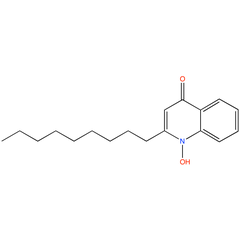
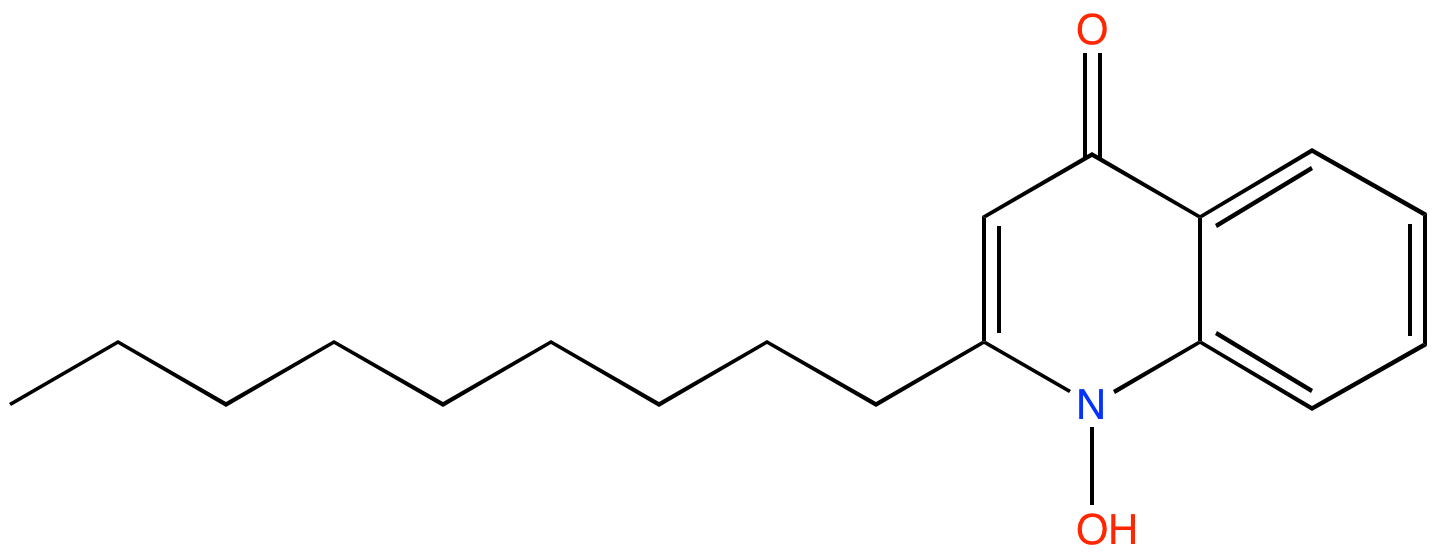
1-Hydroxy-2-nonyl-4(1H)-quinolinone (PAMDB100076)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB100076 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 1-Hydroxy-2-nonyl-4(1H)-quinolinone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 1-Hydroxy-2-nonyl-4(1H)-quinolinone (NQNO) is a C9- congener of HQNO in P. aeruginosa. NQNO is predicted to function similarly to HQNO, an N-oxide ubiquinone analog that inhibits activity of cytochrome b1 complex. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | • 1-Hydroxy-2-nonyl-4(1H)-chinolinon [German] [ACD/IUPAC Name] • 1-Hydroxy-2-nonyl-4(1H)-quinoléinone [French] [ACD/IUPAC Name] • 1-Hydroxy-2-nonyl-4(1H)-quinolinone [ACD/IUPAC Name] • 4(1H)-Quinolinone, 1-hydroxy-2-nonyl- [ACD/Index Name] • 133080-32-7 [RN] • 1-HYDROXY-2-NONYLQUINOLIN-4(1H)-ONE • 2503-85-7 [RN] • 2-Nonyl-4-quinolinol 1-oxide [ACD/IUPAC Name] • 2-Nonylquinolin-4-ol, 1-oxide • 316-66-5 [RN] • 4302-97-0 [RN] • 4663-86-9 [RN] • 4-Quinolinol, 2-nonyl-, 1-oxide [ACD/Index Name] • nonyl-4-hydroxyquinoline-N-oxide • NQNO • NQ-N-Oxide • QNO | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: |
C18H25NO2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 287.397 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 287.188538 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: |
JYFNTDPGDPZMCM-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C18H25NO2/c1-2-3-4-5-6-7-8-11-15-14-18(20)16-12-9-10-13-17(16)19(15)21/h9-10,12-14,21H,2-8,11H2,1H3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 1-hydroxy-2-nonyl-1,4-dihydroquinolin-4-one | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CCCCCCCCCc1cc(=O)c2ccccc2n1O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxyquinolones. These are compounds containing a quinoline moiety bearing a hydroxyl group and a ketone. Quinoline or benzo[b]pyridine is a bicyclic compound that consists of benzene fused to a pyridine. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Quinolines and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Quinolones and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Hydroxyquinolones | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: |
Solid, off white powder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: |
Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) |
Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||