|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100044 |
|---|
|
Identification |
|---|
| Name: |
6-hydroxy-N-methylmyosmine |
|---|
| Description: | A member of the class of pyrrolines that is N-methyl-2-pyrroline carrying a 6-hydroxypyridin-3-yl substituent at position 2. |
|---|
|
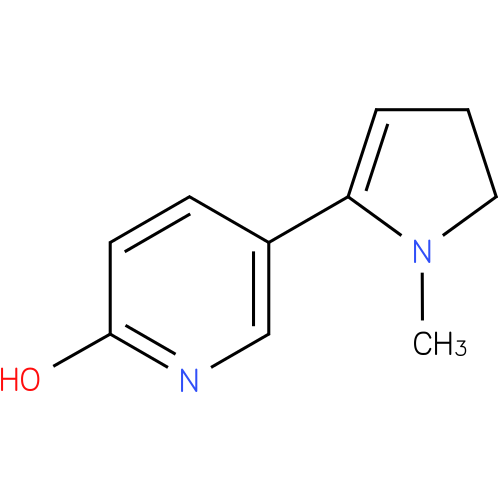
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C10H12N2O |
|---|
| Average Molecular Weight: |
176.2151 |
|---|
| Monoisotopic Molecular
Weight: |
176.095 |
|---|
| InChI Key: |
LWGKCPAYDQWMMS-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H12N2O/c1-12-6-2-3-9(12)8-4-5-10(13)11-7-8/h3-5,7H,2,6H2,1H3,(H,11,13) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 5-(1-methyl-4,5-dihydro-1H-pyrrol-2-yl)pyridin-2(1H)-one |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CN1CCC=C1c1ccc(O)nc1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyridinones. These are compounds containing a pyridine ring, which bears a ketone. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pyridines and derivatives |
|---|
| Sub Class | Hydropyridines |
|---|
|
Direct Parent |
Pyridinones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Dihydropyridine
- Pyridinone
- Pyrroline
- Heteroaromatic compound
- Lactam
- Tertiary amine
- Tertiary aliphatic amine
- Enamine
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- monohydroxypyridine, pyrroline (CHEBI:87460)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Yu H, Tang H, Zhu X, Li Y, Xu P. Molecular mechanism of nicotine degradation
by a newly isolated strain, Ochrobactrum sp. strain SJY1. Appl Environ Microbiol.
2015 Jan;81(1):272-81. Pubmed: 25344232
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|