|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100042 |
|---|
|
Identification |
|---|
| Name: |
furanomycin |
|---|
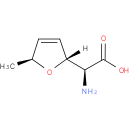
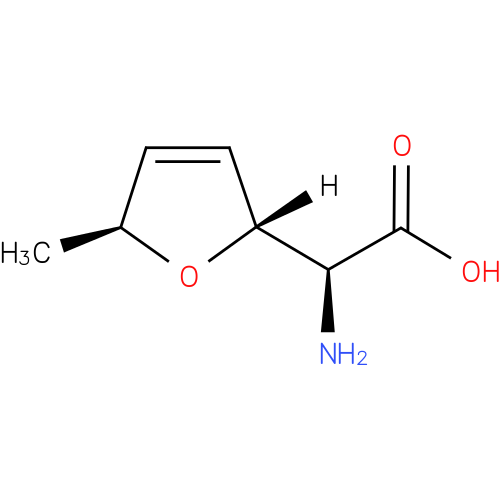
| Description: | A non-proteinogenic L-α-amino acid that is L-alanine in which the methyl group is replaced by a (2R,5S)-5-methyl-2,5-dihydrofuran-2-yl moiety. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (+)-furanomycin
- (2S,2'R,5'S)-2-amino-2-(5'-methyl-2',5'-dihydrofuran-2'-yl)acetic acid
- (6S)-
 2- 2-6(8)7(9)10/h2-6H,8H2,1H3,(H,9,10)/t4-,5+,6-/m0/s1</td></tr><tr><th>CAS
number:</th><td>
<span class='wishart wishart-not-available'>Not Available</span></td></tr><tr><th>IUPAC Name:</th><td>(2<i>S</i>)-amino[(2<i>R</i>,5<i>S</i>)-5-methyl-2,5-dihydrofuran-2-yl]acetic acid</td></tr><tr><th>
Traditional IUPAC Name:</th><td>
<span class='wishart wishart-not-available'>Not Available</span></td></tr><tr><th>SMILES:</th><td>C[C@@H]1O[C@H](C=C1)[C@H](N)C(O)=O</td></tr><tr id=)
Chemical Taxonomy |
|
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
L-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- L-alpha-amino acid
- Dihydrofuran
- Amino acid
- Carboxylic acid
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- non-proteinogenic L-alpha-amino acid, dihydrofuran (CHEBI:79390)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Kohno T, Kohda D, Haruki M, Yokoyama S, Miyazawa T. Nonprotein amino acid
furanomycin, unlike isoleucine in chemical structure, is charged to isoleucine
tRNA by isoleucyl-tRNA synthetase and incorporated into protein. J Biol Chem.
1990 Apr 25;265(12):6931-5. Pubmed: 2182633
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|