|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100023 |
|---|
|
Identification |
|---|
| Name: |
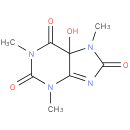
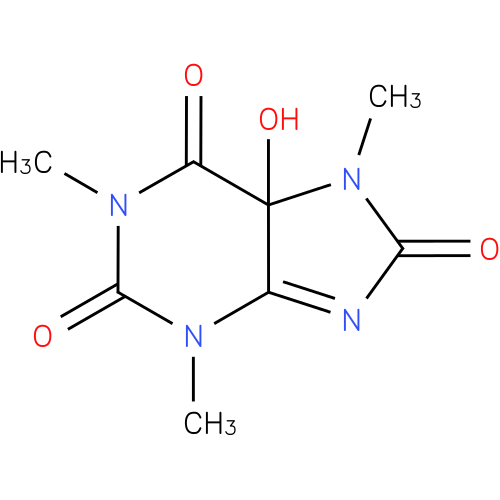
1,3,7-trimethyl-5-hydroxyisouric acid |
|---|
| Description: | A member of the class of oxopurines that is 5-hydroxyisouric acid carrying three additional methyl substituents at positions 1, 3 and 7. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1,3,7-trimethyl-5-hydroxyisourate
|
|---|
|
Chemical Formula: |
C8H10N4O4 |
|---|
| Average Molecular Weight: |
226.19 |
|---|
| Monoisotopic Molecular
Weight: |
226.07 |
|---|
| InChI Key: |
XNXQVRHXDIDGDT-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H10N4O4/c1-10-4-8(16,12(3)6(14)9-4)5(13)11(2)7(10)15/h16H,1-3H3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 5-hydroxy-1,3,7-trimethyl-5,7-dihydro-1H-purine-2,6,8(3H)-trione |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | N1(C(N(C(C2(N(C(N=C12)=O)C)O)=O)C)=O)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
|
Direct Parent |
Xanthines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Xanthine
- Alpha-amino acid or derivatives
- Purinone
- Alkaloid or derivatives
- N-acyl urea
- Pyrimidone
- Ureide
- 1,3-diazinane
- Pyrimidine
- Dicarboximide
- 3-imidazoline
- Carbonic acid derivative
- Urea
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Amidine
- Carboxylic acid amidine
- Carboxylic acid derivative
- Carboximidamide
- Azacycle
- Alkanolamine
- Organic nitrogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Mohanty SK, Yu C-L, Das S, Louie TM, Gakhar L, Subramanian M. Delineation of the Caffeine C-8 Oxidation Pathway in Pseudomonas sp. Strain CBB1 via Characterization of a New Trimethyluric Acid Monooxygenase and Genes Involved in Trimethyluric Acid Metabolism. Journal of Bacteriology. 2012;194(15):3872-3882. Pubmed: 3416557
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|