|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100006 |
|---|
|
Identification |
|---|
| Name: |
syringolin A |
|---|
| Description: | A syrbactin that has a (3E,9E)-2,7-dioxo-1,6-diazacyclododeca-3,9-diene skeleton that is substituted by an isopropyl group at position 5 and by a [(2S)-2-({[(1S)-1-carboxy-2-methylpropyl]carbamoyl}amino)-3-methylbutanoyl]nitril |
|---|
|
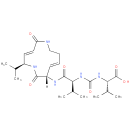
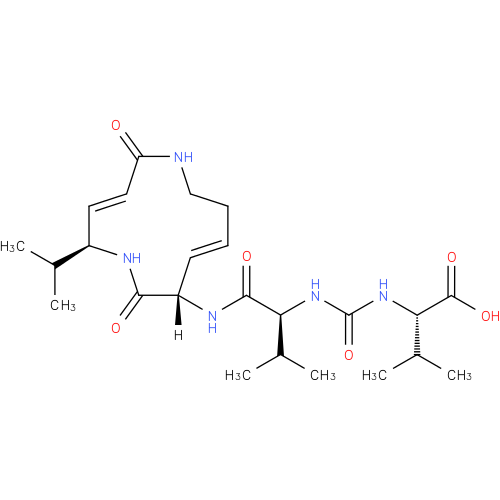
Structure |
|
|---|
| Synonyms: | - DTXSID6037691
- N-[(1-{[(3E,9Z)-5-isopropyl-2,7-dioxo-1,6-diazacyclododeca-3,9-dien-8-yl]carbamoyl}-2-methylpropyl)carbamoyl]valine
|
|---|
|
Chemical Formula: |
C24H39N5O6 |
|---|
| Average Molecular Weight: |
493.5964 |
|---|
| Monoisotopic Molecular
Weight: |
493.29 |
|---|
| InChI Key: |
RUWSLQOIGKYPEZ-YPXRAQKDSA-N |
|---|
| InChI: | InChI=1S/C24H39N5O6/c1-13(2)16-10-11-18(30)25-12-8-7-9-17(21(31)26-16)27-22(32)19(14(3)4)28-24(35)29-20(15(5)6)23(33)34/h7,9-11,13-17,19-20H,8,12H2,1-6H3,(H,25,30)(H,26,31)(H,27,32)(H,33,34)(H2,28,29,35)/b9-7-,11-10+ |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-[[1-[[(3E,9Z)-2,7-dioxo-5-propan-2-yl-1,6-diazacyclododeca-3,9-dien-8-yl]amino]-3-methyl-1-oxobutan-2-yl]carbamoylamino]-3-methylbutanoic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(C)[C@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1\C=C\CCNC(=O)\C=C\[C@@H](NC1=O)C(C)C)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Dipeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-dipeptide
- Valine or derivatives
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- N-carbamoyl-alpha-amino acid
- N-carbamoyl-alpha-amino acid or derivatives
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Branched fatty acid
- Heterocyclic fatty acid
- Methyl-branched fatty acid
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Carbonic acid derivative
- Urea
- Secondary carboxylic acid amide
- Carboxamide group
- Lactam
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Azacycle
- Carboxylic acid
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- ureas, monocarboxylic acid, homodetic cyclic peptide, syrbactin (CHEBI:80033)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Clerc J, Florea BI, Kraus M, Groll M, Huber R, Bachmann AS, Dudler R, Driessen
C, Overkleeft HS, Kaiser M. Syringolin A selectively labels the 20 S proteasome
in murine EL4 and wild-type and bortezomib-adapted leukaemic cell lines.
Chembiochem. 2009 Nov 2;10(16):2638-43. Pubmed: 19746508
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|