|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004741 |
|---|
|
Identification |
|---|
| Name: |
N-Acetylphosphinothricin |
|---|
| Description: | N-Acetylphosphinothricin is an intermediate in phosphonate and phosphinate metabolism in E.coli, where the enzyme acetyl-CoA:phosphinothricin N-acetyltransferase catalyzes the reaction acetyl-CoA + phosphinothricin <=> CoA + N-acetylphosphinothricin (KEGG compound: C17952). |
|---|
|
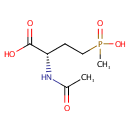
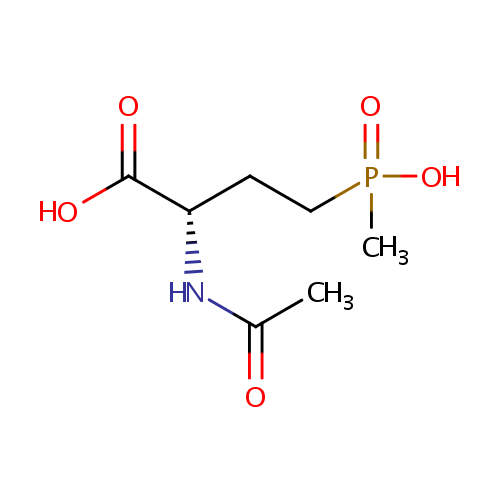
Structure |
|
|---|
| Synonyms: | - L-N-Acetylphosphinothricin
- N-Acetyl-L-glufosinate
- N-Acetyl-L-glufosinic acid
- N-Acetylphinothricin
|
|---|
|
Chemical Formula: |
C7H14NO5P |
|---|
| Average Molecular Weight: |
223.165 |
|---|
| Monoisotopic Molecular
Weight: |
223.060959553 |
|---|
| InChI Key: |
VZVQOWUYAAWBCP-LURJTMIESA-N |
|---|
| InChI: | InChI=1S/C7H14NO5P/c1-5(9)8-6(7(10)11)3-4-14(2,12)13/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)(H,12,13)/t6-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2S)-2-acetamido-4-[hydroxy(methyl)phosphoryl]butanoic acid |
|---|
|
Traditional IUPAC Name: |
(2S)-2-acetamido-4-[hydroxy(methyl)phosphoryl]butanoic acid |
|---|
| SMILES: | CC(=O)N[C@@H](CCP(C)(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl-aliphatic-alpha amino acids. These are alpha amino acids carrying a N-acylated aliphatic chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
N-acyl-aliphatic-alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-l-alpha-amino acid
- N-acyl-aliphatic-alpha amino acid
- Amino fatty acid
- Fatty acyl
- Fatty acid
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid amide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Organophosphorus compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Phosphonate and phosphinate metabolism pae00440
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 14647097 | | Kegg ID | C17952 | | ChemSpider ID | 30791551 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|