|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004379 |
|---|
|
Identification |
|---|
| Name: |
L-Valyl-tRNA(Val) |
|---|
| Description: | L-Valyl-tRNA(Val) is an intermediate in tRNA charging pathway in E.coli. It is a product for the enzyme valyl-tRNA synthetase which catalyzes the reaction a tRNAval + L-valine + ATP + H+ -> an L-valyl-[tRNAval] + AMP + diphosphate (BioCyc class: Charged-LYS-tRNAs). |
|---|
|
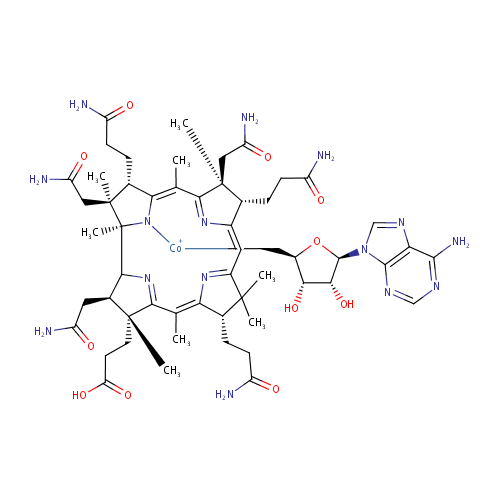
Structure |
|
|---|
| Synonyms: | - Adenosyl cobyrinate hexaamide
- Adenosyl cobyrinic acid hexaamide
- Adenosylcobyrate
- Adenosylcobyric acid
|
|---|
|
Chemical Formula: |
C55H77CoN15O11 |
|---|
| Average Molecular Weight: |
1183.248 |
|---|
| Monoisotopic Molecular
Weight: |
1182.525344 |
|---|
| InChI Key: |
AXZSUSWNAXMBBB-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C45H66N10O8.C10H12N5O3.Co/c1-21-36-24(10-13-30(47)57)41(3,4)28(53-36)18-27-23(9-12-29(46)56)43(6,19-33(50)60)39(52-27)22(2)37-25(11-14-31(48)58)44(7,20-34(51)61)45(8,55-37)40-26(17-32(49)59)42(5,38(21)54-40)16-15-35(62)63;1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15;/h18,23-26,40H,9-17,19-20H2,1-8H3,(H14,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63);2-4,6-7,10,16-17H,1H2,(H2,11,12,13);/q;;+2/p-1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | {[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}[(1R,3R,4R,8S,13S,14S,18S,19S)-8,13,18-tris(2-carbamoylethyl)-3,14,19-tris(carbamoylmethyl)-4-(2-carboxyethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium |
|---|
|
Traditional IUPAC Name: |
{[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}[(1R,3R,4R,8S,13S,14S,18S,19S)-8,13,18-tris(2-carbamoylethyl)-3,14,19-tris(carbamoylmethyl)-4-(2-carboxyethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium |
|---|
| SMILES: | [Co++].[H]C1([CH2])OC([H])(N2C=NC3=C(N)N=CN=C23)C([H])(O)C1([H])O.[H]C1(CCC([NH-])=O)\C2=C\C3=N\C(=C(C)\C4=NC([H])(C([H])(CC(O)=N)C4(C)CCC(O)=O)C4(C)N\C(=C(C)/C(=N2)C1(C)CC(O)=N)C([H])(CCC(O)=N)C4(C)CC(O)=N)\C([H])(CCC(O)=N)C3(C)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | Not Available |
|---|
|
Kingdom |
Not Available |
|---|
| Super Class | Not Available |
|---|
|
Class |
Not Available |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Not Available |
|---|
| Alternative Parents |
Not Available |
|---|
| Substituents |
Not Available |
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Aminoacyl-tRNA biosynthesis pae00970
- Valine, leucine and isoleucine biosynthesis pae00290
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | C02554 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|