|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004290 |
|---|
|
Identification |
|---|
| Name: |
L-Glutamyl-tRNA(Glu) |
|---|
| Description: | This list contains a list of EC numbers for the fourth group, EC 4, lyases, placed in numerical order as determined by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. |
|---|
|
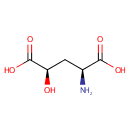
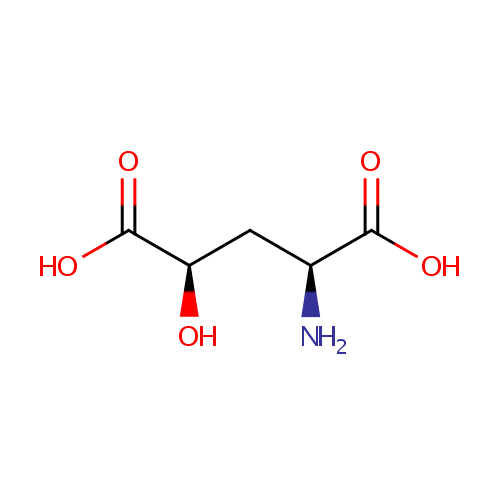
Structure |
|
|---|
| Synonyms: | - (2S,4R)-2-amino-4-Hydroxypentanedioate
- (2S,4R)-2-amino-4-Hydroxypentanedioic acid
- L-erythro-4-Hydroxyglutamate
- L-erythro-4-Hydroxyglutamic acid
|
|---|
|
Chemical Formula: |
C5H9NO5 |
|---|
| Average Molecular Weight: |
163.1287 |
|---|
| Monoisotopic Molecular
Weight: |
163.048072403 |
|---|
| InChI Key: |
HBDWQSHEVMSFGY-STHAYSLISA-N |
|---|
| InChI: | InChI=1S/C5H9NO5/c6-2(4(8)9)1-3(7)5(10)11/h2-3,7H,1,6H2,(H,8,9)(H,10,11)/t2-,3+/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2S,4R)-2-amino-4-hydroxypentanedioic acid |
|---|
|
Traditional IUPAC Name: |
(2S,4R)-2-amino-4-hydroxypentanedioic acid |
|---|
| SMILES: | N[C@@H](C[C@@H](O)C(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxy fatty acids. These are fatty acids in which the chain bears a hydroxyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Hydroxy fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxy fatty acid
- L-alpha-amino acid
- Alpha-amino acid or derivatives
- Alpha-amino acid
- Short-chain hydroxy acid
- Amino fatty acid
- Hydroxy acid
- Dicarboxylic acid or derivatives
- Alpha-hydroxy acid
- 1,3-aminoalcohol
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 21285 | | HMDB ID | Not Available | | Pubchem Compound ID | 440854 | | Kegg ID | C02987 | | ChemSpider ID | 389696 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|