|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003576 |
|---|
|
Identification |
|---|
| Name: |
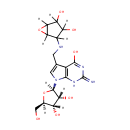
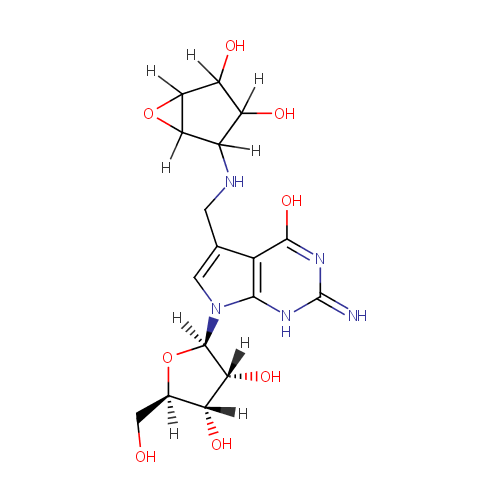
epoxyqueuosine |
|---|
| Description: | Epoxyqueuosine is an intermediate in queosine biosynthesis. Queuosine is an important 7-deazapurine-modified nucleoside that is present in certain tRNAs in bacteria and most eukaryotes. Eposyqueosiine is a subsstrate for Epoxyqueuosine reductase catalyzes the final step in the de novo synthesis of queuosine, the anticodon loop modification found in tRNA(Asp), tRNA(Asn), tRNA(His), and tRNA(Tyr) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-amino-7-(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl-5-(3,4-dihydroxy-6-oxabicyclo3.1.0hexan-2-yl)aminomethyl-1H-pyrrolo2,3-dpyrimidin-4-one
|
|---|
|
Chemical Formula: |
C17H23N5O8 |
|---|
| Average Molecular Weight: |
425.3932 |
|---|
| Monoisotopic Molecular
Weight: |
425.154662737 |
|---|
| InChI Key: |
RRCFLRBBBFZLSB-MPMHWICOSA-N |
|---|
| InChI: | InChI=1S/C17H23N5O8/c18-17-20-14-6(15(28)21-17)4(1-19-7-9(25)10(26)13-12(7)30-13)2-22(14)16-11(27)8(24)5(3-23)29-16/h2,5,7-13,16,19,23-27H,1,3H2,(H3,18,20,21,28)/t5-,7?,8-,9?,10?,11-,12?,13?,16-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 4-[({7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-2-imino-1H,2H,7H-pyrrolo[2,3-d]pyrimidin-5-yl}methyl)amino]-6-oxabicyclo[3.1.0]hexane-2,3-diol |
|---|
|
Traditional IUPAC Name: |
4-[({7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-2-imino-1H-pyrrolo[2,3-d]pyrimidin-5-yl}methyl)amino]-6-oxabicyclo[3.1.0]hexane-2,3-diol |
|---|
| SMILES: | [H]C12OC1([H])C([H])(NCC1=CN(C3=C1C(O)=NC(=N)N3)[C@]1([H])O[C@]([H])(CO)[C@@]([H])(O)[C@@]1([H])O)C([H])(O)C2([H])O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrrolopyrimidine nucleosides and nucleotides. These are nucleoside derivatives containing a ribose derivative which is n-glycosylated to a pyrrolopyrimidine. Also called deazapurine nucleosides, they are analogs of purine nucleosides with the N atom of the purine being replaced by a C atom at position 7. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrrolopyrimidine nucleosides and nucleotides |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Pyrrolopyrimidine nucleosides and nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrrolopyrimidine ribonucleoside
- N-glycosyl compound
- Glycosyl compound
- Pyrrolopyrimidine
- Hydroxypyrimidine
- Aralkylamine
- Substituted pyrrole
- Pyrimidine
- Oxane
- Monosaccharide
- Heteroaromatic compound
- Pyrrole
- Oxolane
- Cyclic alcohol
- Secondary alcohol
- 1,2-diol
- 1,2-aminoalcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Ether
- Oxirane
- Secondary aliphatic amine
- Dialkyl ether
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 3035951 | | Kegg ID | Not Available | | ChemSpider ID | 2300068 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|