|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003492 |
|---|
|
Identification |
|---|
| Name: |
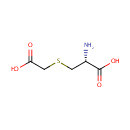
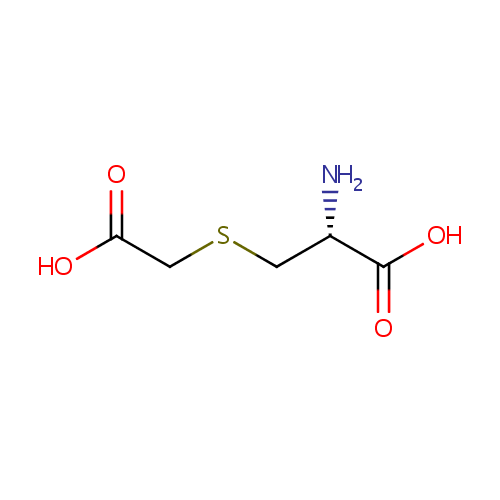
S-Carboxymethyl-L-cysteine |
|---|
| Description: | S-Carboxymethyl-L-cysteine is the side-chain carboxymethyl derivative of cysteine. It is produced during the metabolism of 3-chloro-L-alanine via the enzyme Cysteine synthase A. The reaction is 3-chloro-alanine + thioglycolate = S-carboxymethyl-L-cysteine + chloride. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2R)-2-amino-3-(Carboxymethylsulfanyl)propanoate
- (2R)-2-amino-3-(carboxymethylsulfanyl)propanoic acid
- (2R)-2-amino-3-(Carboxymethylsulphanyl)propanoate
- (2R)-2-amino-3-(Carboxymethylsulphanyl)propanoic acid
- (L)-2-amino-3-(carboxymethylthio)Propionate
- (L)-2-Amino-3-(carboxymethylthio)propionic acid
- (R)-S-(carboxymethyl)cysteine
- 1-Carboxymethylcysteine
- 2-amino-3-(carboxymethylthio)Propionate
- 2-Amino-3-(carboxymethylthio)propionic acid
- 2-amino-3-[(Carboxymethyl)sulfanyl]propanoate
- 2-amino-3-[(carboxymethyl)sulfanyl]propanoic acid
- 2-amino-3-[(Carboxymethyl)sulphanyl]propanoate
- 2-amino-3-[(Carboxymethyl)sulphanyl]propanoic acid
- 3-((Carboxymethyl)thio)alanine
- 3-(Carboxymethylthio)-L-alanine
- 3-(Carboxymethylthio)alanine
- 3-[(Carboxymethyl)thio]-L-alanine
- 3-[(Carboxymethyl)thio]alanine
- 5-amino-3-Thiadihexanoate
- 5-Amino-3-thiadihexanoic acid
- Carbocisteine
- Carbocysteine
- Carboxymethylated cysteine
- Carboxymethylcysteine
- Carboxymethylenecysteine
- L-3-((carboxymethyl)thio)alanine
- L-Carbocisteine
- L-Carboxymethylcysteine
- L-form
- Loviscol
- Muciclar
- Mucocis
- Mucodine
- Mucodyne
- Mucofan
- Reomucil
- Rhinathiol
- S-(carboxymethyl)-(R)-cysteine
- S-(carboxymethyl)-L-cysteine
- S-(Carboxymethyl)cysteine
- S-Carboxylmethyl-L-cysteine
- S-Carboxymethylcysteine
- S-Carboxymethylcysteine, 9CI
- Thiodril
|
|---|
|
Chemical Formula: |
C5H9NO4S |
|---|
| Average Molecular Weight: |
179.194 |
|---|
| Monoisotopic Molecular
Weight: |
179.025228471 |
|---|
| InChI Key: |
GBFLZEXEOZUWRN-VKHMYHEASA-N |
|---|
| InChI: | InChI=1S/C5H9NO4S/c6-3(5(9)10)1-11-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 |
|---|
| CAS
number: |
2387-59-9 |
|---|
| IUPAC Name: | (2R)-2-amino-3-[(carboxymethyl)sulfanyl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
carbocisteine |
|---|
| SMILES: | N[C@@H](CSCC(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as s-alkyl-l-cysteines. These are cysteine derivatives that carry an alkyl chain attached to the sulfanyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
S-alkyl-L-cysteines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- S-alkyl-l-cysteine
- Dicarboxylic acid or derivatives
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Carboxylic acid
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
204 - 207 C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|