|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003406 |
|---|
|
Identification |
|---|
| Name: |
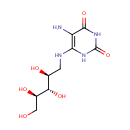
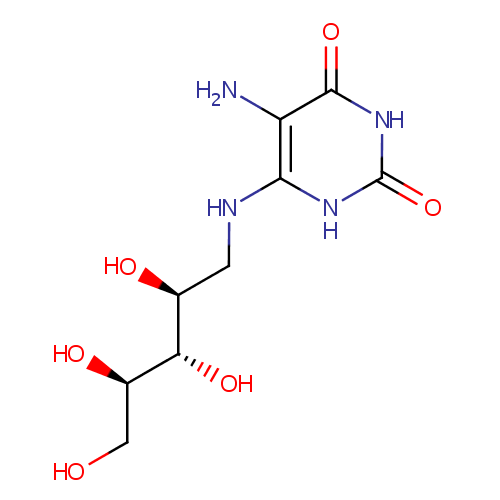
5-amino-6-(D-ribitylamino)uracil |
|---|
| Description: | 5-amino-6-(D-ribitylamino)uracil is involved in the Cofactor riboflavin biosynthesis pathway. It is a substrate of Riboflavin biosynthesis protein RibD that catalyses the following reactions: 2-hydroxy-3-oxobutyl phosphate + 5-amino-6-(D-ribitylamino)uracil => 6,7-dimethyl-8-(1-D-ribityl)lumazine + H(2)O + 1 phosphate and 6,7-dimethyl-8-(1-D-ribityl)lumazine => 5-amino-6-(D-ribitylamino)uracil + riboflavin |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1-(5-Amino-2,6-dioxo-1,2,3,6-tetrahydro-4-pyrimidinyl)amino-1-deoxy-D-ribitol
- 4-(1-D-Ribitylamino)-5-amino-2,6-dihydroxypyrimidine
- 5-amino-2,6-dioxo-4-ribitylaminopyrimidine
- 5-Amino-6-(1-D-ribitylamino)uracil
- 5-amino-6-(2S,3S,4R)-2,3,4,5-tetrahydroxypentylamino-1H-pyrimidine-2,4-dione
- 5-amino-6-ribitylamino-2,4-(1H,3H)pyrimidinedione
- 5-Amino-6-ribitylaminouracil
- 5-arpd
- 6-(1-D-Ribitylamino)-5-amino-2,4-dihydroxypyrimidine
- 6-(1-D-Ribitylamino)-5-aminouracil
|
|---|
|
Chemical Formula: |
C9H16N4O6 |
|---|
| Average Molecular Weight: |
276.2465 |
|---|
| Monoisotopic Molecular
Weight: |
276.106984264 |
|---|
| InChI Key: |
XKQZIXVJVUPORE-RPDRRWSUSA-N |
|---|
| InChI: | InChI=1S/C9H16N4O6/c10-5-7(12-9(19)13-8(5)18)11-1-3(15)6(17)4(16)2-14/h3-4,6,14-17H,1-2,10H2,(H3,11,12,13,18,19)/t3-,4+,6-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 5-amino-6-{[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]amino}-1,2,3,4-tetrahydropyrimidine-2,4-dione |
|---|
|
Traditional IUPAC Name: |
5-arpd |
|---|
| SMILES: | NC1=C(NC[C@H](O)[C@H](O)[C@H](O)CO)NC(=O)NC1=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxypyrimidines. These are organic compounds containing a hydroxyl group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
|
Direct Parent |
Hydroxypyrimidines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxypyrimidine
- Secondary aliphatic/aromatic amine
- Aminopyrimidine
- Imidolactam
- Primary aromatic amine
- Saccharide
- Heteroaromatic compound
- Secondary alcohol
- Polyol
- 1,2-diol
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 15934 | | HMDB ID | Not Available | | Pubchem Compound ID | 193516 | | Kegg ID | C04732 | | ChemSpider ID | 167930 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|