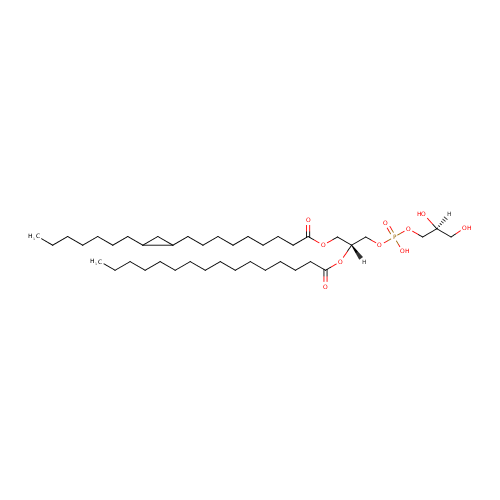
PG(16:0/19:0cycw8c) (PAMDB002033)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB002033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | PG(16:0/19:0cycw8c) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | PG(16:0/19:0cycw8c) is a phosphatidylglycerol. Phosphatidylglycerols consist of a glycerol 3-phosphate backbone esterified to either saturated or unsaturated fatty acids on carbons 1 and 2. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PG(16:0/19:0cycw8c), in particular, consists of one hexadecanoyl chain to the C-1 atom, and one 9-(2-heptylcyclopropyl)nonanoyl to the C-2 atom. In Pseudomonas aeruginosa glycerophospholipid metabolism, phosphatidylglycerol is formed from phosphatidic acid (1,2-diacyl-sn-glycerol 3-phosphate) by a sequence of enzymatic reactions that proceeds via two intermediates, cytidine diphosphate diacylglycerol (CDP-diacylglycerol) and phosphatidylglycerophosphate (PGP, a phosphorylated phosphatidylglycerol). Phosphatidylglycerols, along with CDP-diacylglycerol, also serve as precursor molecules for the synthesis of cardiolipin, a phospholipid found in membranes. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C41H79O10P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 763.047 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 762.541085742 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | XLFQKCMCWQYOHD-NIJRPICPSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C41H79O10P/c1-3-5-7-9-10-11-12-13-14-15-16-22-26-30-41(45)51-39(35-50-52(46,47)49-33-38(43)32-42)34-48-40(44)29-25-21-18-17-20-24-28-37-31-36(37)27-23-19-8-6-4-2/h36-39,42-43H,3-35H2,1-2H3,(H,46,47)/t36?,37?,38-,39+/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [(2S)-2,3-dihydroxypropoxy][(2R)-3-{[9-(2-heptylcyclopropyl)nonanoyl]oxy}-2-(hexadecanoyloxy)propoxy]phosphinic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (2S)-2,3-dihydroxypropoxy((2R)-3-{[9-(2-heptylcyclopropyl)nonanoyl]oxy}-2-(hexadecanoyloxy)propoxy)phosphinic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@](O)(CO)COP(O)(=O)OC[C@@]([H])(COC(=O)CCCCCCCCC1CC1CCCCCCC)OC(=O)CCCCCCCCCCCCCCC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as phosphatidylglycerols. These are glycerophosphoglycerols in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Glycerophospholipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Glycerophosphoglycerols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Phosphatidylglycerols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | PGP(16:0/18:1(11Z)) + Water > PG(16:0/19:0cycw8c) + Phosphate PE(17:0cycw7c/17:0cycw7c) + PG(16:0/19:0cycw8c) > CL(16:0/17:0cycw7c/17:0cycw7c/19:0cycv8c) + Ethanolamine PE(17:0cycw7c/17:0cycw7c) + PG(16:0/19:0cycw8c) > Ethanolamine + CL(16:0/19:0cycv8c/17:0cycw7c/17:0cycw7c) PE(17:0cycw7c/17:0cycw7c) + PG(16:0/19:0cycw8c) > CL(17:0cycw7c/16:0/17:0cycw7c/19:0cycv8c) + Ethanolamine PE(17:0cycw7c/17:0cycw7c) + PG(16:0/19:0cycw8c) > Ethanolamine + CL(17:0cycw7c/16:0/19:0cycv8c/17:0cycw7c) PE(17:0cycw7c/17:0cycw7c) + PG(16:0/19:0cycw8c) > Ethanolamine + CL(19:0cycv8c/16:0/17:0cycw7c/17:0cycw7c) PE(14:0(3-OH)/19:0cycv8c) + PG(16:0/19:0cycw8c) > Ethanolamine + CL(19:0cycv8c/16:0/14:0/19:0cycv8c) PE(14:0/14:0) + PG(16:0/19:0cycw8c) > Ethanolamine + CL(19:0cycv8c/16:0/14:0/14:0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in phosphatidylglycerophosphatase activity
- Specific function:
- One of the three phospholipid phosphatases, specifically hydrolyzes phosphatidylglycerophosphate
- Gene Name:
- pgpA
- Locus Tag:
- PA4050
- Molecular weight:
- 19.6 kDa
Reactions
| Phosphatidylglycerophosphate + H(2)O = phosphatidylglycerol + phosphate. |
- General function:
- Involved in phosphotransferase activity, for other substituted phosphate groups
- Specific function:
- Catalyzes the reversible phosphatidyl group transfer from one phosphatidylglycerol molecule to another to form cardiolipin (CL) (diphosphatidylglycerol) and glycerol. Affects resistance to the gyrase inhibitor novobiocin
- Gene Name:
- cls
- Locus Tag:
- PA5394
- Molecular weight:
- 54.6 kDa
Reactions
| 2 Phosphatidylglycerol = diphosphatidylglycerol + glycerol. |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes, in vitro, the phosphatidyl group transfer from one phosphatidylglycerol molecule to another to form cardiolipin (CL) (diphosphatidylglycerol) and glycerol. Can also catalyze phosphatidyl group transfer to water to form phosphatidate. Catalyzes little, if any, cardiolipin synthesis in vivo, even when the expression level is very high
- Gene Name:
- ybhO
- Locus Tag:
- PA2155
- Molecular weight:
- 46.5 kDa
Reactions
| 2 Phosphatidylglycerol = diphosphatidylglycerol + glycerol. |
Transporters
- General function:
- Secondary metabolites biosynthesis, transport and catabolism
- Specific function:
- Part of the ABC transporter complex mlaFEDB that actively prevents phospholipid accumulation at the cell surface. Probably maintains lipid asymmetry in the outer membrane by retrograde trafficking of phospholipids from the outer membrane to the inner membrane
- Gene Name:
- mlaE
- Locus Tag:
- PA4455
- Molecular weight:
- 28.4 kDa