|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001967 |
|---|
|
Identification |
|---|
| Name: |
Thioglycolate |
|---|
| Description: | Thioglycolic acid (TGA) is the organic compound HSCH2CO2H. It contains both a thiol (mercaptan) and a carboxylic acid. It is a clear liquid with a strong unpleasant odor. It is readily oxidized by air to the corresponding disulfide [SCH2CO2H]2. It is use during the conversion of chloro-alanine to S-carboxymethyl-L-cysteine. |
|---|
|
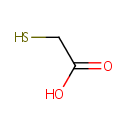
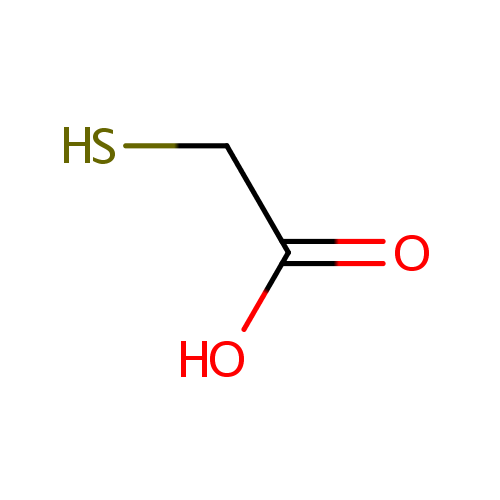
Structure |
|
|---|
| Synonyms: | - Mercaptoacetate
- Mercaptoacetic acid
- Mercaptoethanoate
- Mercaptoethanoic acid
- Sulfanylacetate
- Sulfanylacetic acid
- Sulphanylacetate
- Sulphanylacetic acid
- Thioglycolate
- Thioglycolic acid
|
|---|
|
Chemical Formula: |
C2H4O2S |
|---|
| Average Molecular Weight: |
92.117 |
|---|
| Monoisotopic Molecular
Weight: |
91.993200062 |
|---|
| InChI Key: |
CWERGRDVMFNCDR-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C2H4O2S/c3-2(4)1-5/h5H,1H2,(H,3,4) |
|---|
| CAS
number: |
513-66-6 |
|---|
| IUPAC Name: | 2-sulfanylacetic acid |
|---|
|
Traditional IUPAC Name: |
thioglycolic acid |
|---|
| SMILES: | OC(=O)CS |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alpha-mercaptocarboxylic acids. These are carboxylic acids that bear a thiol group at the C-2 position. Alpha-mercaptocarboxylic acids have the general formula RC(S)C(=O)O, where R = H, organyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acids |
|---|
|
Direct Parent |
alpha-Mercaptocarboxylic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 2-mercaptocarboxylic acid
- Monocarboxylic acid or derivatives
- Alkylthiol
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
-16 ?C, 257 K, 3 ?F |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|