|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001868 |
|---|
|
Identification |
|---|
| Name: |
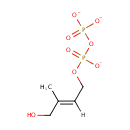
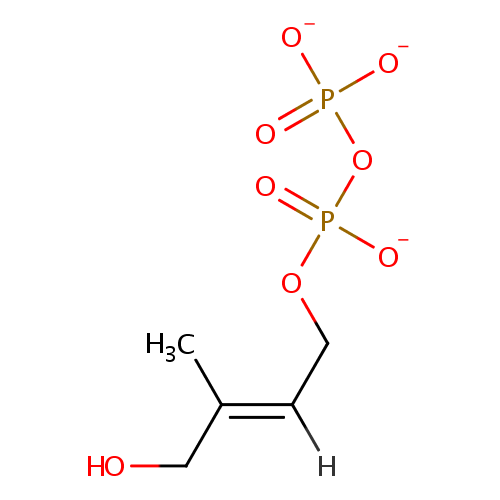
1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate |
|---|
| Description: | 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate is a member of the chemical class known as Organic Pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate is an intermediate in the mehtylerithritol phosphate pathway. . D-glyceraldehyde-3-phosphate and pyruvate are initially combined to yield 1-deoxy-D-xylylose 5-phosphate (DXP). DXP is then rearranged and reduced to generate the pathway's namesake compound, 2-C-methyl-D-erythritol 4-phosphate (MEP). In the third reaction MEP is converted into 4-diphosphocytidyl-2-C-methylerythritol, which is subsequently phosphorylated at the 2 position hydroxy group, yielding 4-diphosphocytidyl-2C-methylerythritol 2-phosphate. This product is then converted into 2-C-methyl-D-erythritol 2,4-cyclodiphosphate. This compound is then reduced to generate 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate. In the final step, this diphosphate compound is converted by a single enzyme into a 5-6:1 ratio of IPP and DMAPP This ratio is subsequently adjusted to 7:3 by isopentenyl diphosphate isomerase. Both IPP and DMAPP then become the basic building blocks of polyisoprenoid biosynthesis. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphoric acid
|
|---|
|
Chemical Formula: |
C5H9O8P2 |
|---|
| Average Molecular Weight: |
259.0677 |
|---|
| Monoisotopic Molecular
Weight: |
258.977265288 |
|---|
| InChI Key: |
MDSIZRKJVDMQOQ-GORDUTHDSA-K |
|---|
| InChI: | InChI=1S/C5H12O8P2/c1-5(4-6)2-3-12-15(10,11)13-14(7,8)9/h2,6H,3-4H2,1H3,(H,10,11)(H2,7,8,9)/p-3/b5-2+ |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | {[(2E)-4-hydroxy-3-methylbut-2-en-1-yl phosphonato]oxy}phosphonate |
|---|
|
Traditional IUPAC Name: |
hmbpp |
|---|
| SMILES: | [H]\C(COP([O-])(=O)OP([O-])([O-])=O)=C(\C)CO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as isoprenoid phosphates. These are prenol lipids containing a phosphate group linked to an isoprene (2-methylbuta-1,3-diene) unit. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Isoprenoid phosphates |
|---|
|
Direct Parent |
Isoprenoid phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Organic pyrophosphate
- Isoprenoid phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 128753 | | HMDB ID | Not Available | | Pubchem Compound ID | 21597501 | | Kegg ID | Not Available | | ChemSpider ID | 10224038 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|