|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001828 |
|---|
|
Identification |
|---|
| Name: |
Adenosine tetraphosphate |
|---|
| Description: | Adenosine 5' tetraphosphate, Ap4, is a natural nucleotide present in many biological systems. Technically adenosine tetraphosphate is condensation product of adenosine with tetraphosphoric acid at the 5' position. Acetyl coenzyme A (CoA) synthetase (EC 6.2.1.1) catalyzes the synthesis of adenosine 5'-tetraphosphate (P4A) and adenosine 5'-pentaphosphate (p5A) from ATP and tri- or tetrapolyphosphate (P3 or P4). [PMID:9620965] |
|---|
|
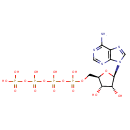
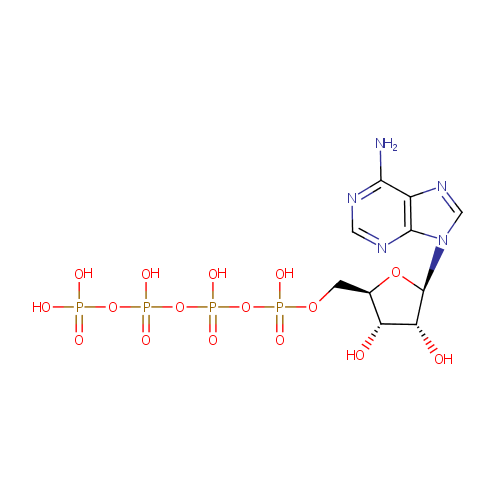
Structure |
|
|---|
| Synonyms: | - Adenosine 5'-tetraphosphate
- Adenosine 5'-tetraphosphoric acid
- Adenosine tetraphosphoric acid
- Ap4
- ATPP
- P4A
|
|---|
|
Chemical Formula: |
C10H17N5O16P4 |
|---|
| Average Molecular Weight: |
587.1609 |
|---|
| Monoisotopic Molecular
Weight: |
586.962075569 |
|---|
| InChI Key: |
WWMWAMFHUSTZTA-KQYNXXCUSA-N |
|---|
| InChI: | InChI=1S/C10H17N5O16P4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(28-10)1-27-33(21,22)30-35(25,26)31-34(23,24)29-32(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H,25,26)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
58337-43-2 |
|---|
| IUPAC Name: | {[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
adenosine tetraphosphic acid |
|---|
| SMILES: | NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside polyphosphates. These are purine ribobucleotides with polyphosphate (with 4 or more phosphate) group linked to the ribose moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside polyphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside polyphosphate
- N-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- Monosaccharide
- Saccharide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- D-Glutamine and D-glutamate metabolism pae00471
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Fontes, R., Sillero, M. A., Sillero, A. (1998). "Acyl coenzyme A synthetase from Pseudomonas fragi catalyzes the synthesis of adenosine 5'-polyphosphates and dinucleoside polyphosphates." J Bacteriol 180:3152-3158. Pubmed: 9620965
- Lazewska D, Starzynska E, Guranowski A: Human placental (Asymmetrical) diadenosine 5',5'''-P1,P4-tetraphosphate hydrolase: purification to homogeneity and some properties. Protein Expr Purif. 1993 Feb;4(1):45-51. Pubmed: 8381042
- Lee JW, Kong ID, Park KS, Jeong SW: Effects of adenosine tetraphosphate (ATPP) on vascular tone in the isolated rat aorta. Yonsei Med J. 1995 Dec;36(6):487-96. Pubmed: 8599250
- Liu Q, Dumont DJ: Molecular cloning and chromosomal localization in human and mouse of the SH2-containing inositol phosphatase, INPP5D (SHIP). Amgen EST Program. Genomics. 1997 Jan 1;39(1):109-12. Pubmed: 9027494
- Luthje J, Baringer J, Ogilvie A: Effects of diadenosine triphosphate (Ap3A) and diadenosine tetraphosphate (Ap4A) on platelet aggregation in unfractionated human blood. Blut. 1985 Dec;51(6):405-13. Pubmed: 3852686
- Pintor J, Carracedo G, Alonso MC, Bautista A, Peral A: Presence of diadenosine polyphosphates in human tears. Pflugers Arch. 2002 Jan;443(3):432-6. Epub 2001 Aug 23. Pubmed: 11810214
- Pintor J, Pelaez T, Peral A: Adenosine tetraphosphate, Ap4, a physiological regulator of intraocular pressure in normotensive rabbit eyes. J Pharmacol Exp Ther. 2004 Feb;308(2):468-73. Epub 2003 Nov 4. Pubmed: 14600249
- Pintor J, Peral A, Hoyle CH, Redick C, Douglass J, Sims I, Yerxa B: Effects of diadenosine polyphosphates on tear secretion in New Zealand white rabbits. J Pharmacol Exp Ther. 2002 Jan;300(1):291-7. Pubmed: 11752128
- Sillero MA, Del Valle M, Zaera E, Michelena P, Garcia AG, Sillero A: Diadenosine 5',5"-P1,P4-tetraphosphate (Ap4A), ATP and catecholamine content in bovine adrenal medulla, chromaffin granules and chromaffin cells. Biochimie. 1994;76(5):404-9. Pubmed: 7849106
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|