|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001609 |
|---|
|
Identification |
|---|
| Name: |
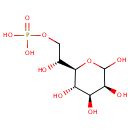
D-Glycero-D-manno-heptose 7-phosphate |
|---|
| Description: | D-glycero-D-manno-heptose 7-phosphate is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 7-O-Phosphono-D-glycero-D-manno-heptose
- D-α,β-D-heptose-7-phosphate
- D-α,β-D-heptose-7-phosphoric acid
- D-glycero-α,β-D-manno-heptose 7-P
- D-Glycero-α,β-D-manno-heptose 7-P
- D-glycero-b-D-manno-Heptose 7-phosphate
- D-glycero-b-D-manno-Heptose 7-phosphoric acid
- D-Glycero-beta-D-manno-heptose 7-phosphate
- D-glycero-beta-D-manno-Heptose 7-phosphoric acid
- D-glycero-D-heptose 7-P
- D-Glycero-D-manno-heptose 7-(dihydrogen phosphate)
- D-glycero-D-manno-Heptose 7-(dihydrogen phosphoric acid)
- D-Glycero-D-manno-Heptose 7-phosphate
- D-glycero-D-manno-Heptose 7-phosphoric acid
- D-glycero-β-D-manno-Heptose 7-phosphate
- D-glycero-β-D-manno-Heptose 7-phosphoric acid
|
|---|
|
Chemical Formula: |
C7H15O10P |
|---|
| Average Molecular Weight: |
290.1618 |
|---|
| Monoisotopic Molecular
Weight: |
290.040283212 |
|---|
| InChI Key: |
SDADNVAZGVDAIM-NNPWBXLPSA-N |
|---|
| InChI: | InChI=1S/C7H15O10P/c8-2(1-16-18(13,14)15)6-4(10)3(9)5(11)7(12)17-6/h2-12H,1H2,(H2,13,14,15)/t2-,3+,4+,5+,6-,7?/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(2R)-2-hydroxy-2-[(2R,3S,4S,5S)-3,4,5,6-tetrahydroxyoxan-2-yl]ethoxy]phosphonic acid |
|---|
|
Traditional IUPAC Name: |
(2R)-2-hydroxy-2-[(2R,3S,4S,5S)-3,4,5,6-tetrahydroxyoxan-2-yl]ethoxyphosphonic acid |
|---|
| SMILES: | O[C@H](COP(O)(O)=O)[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organophosphorus compounds |
|---|
|
Class |
Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
|
Direct Parent |
Monoalkyl phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monoalkyl phosphate
- Oxane
- Organic phosphate
- Monosaccharide
- Secondary alcohol
- Polyol
- Hemiacetal
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|