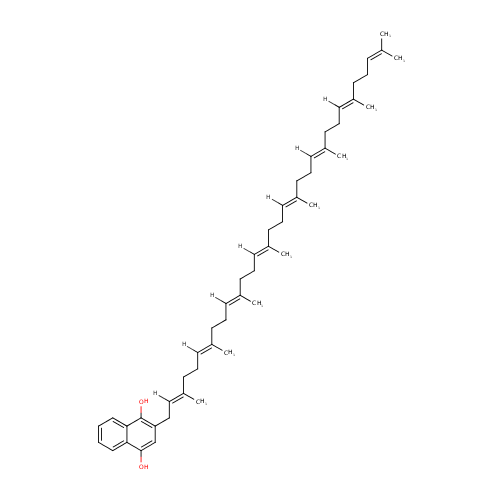
2-Demethylmenaquinol 8 (PAMDB001563)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001563 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2-Demethylmenaquinol 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2-demethylmenaquinol 8 belongs to the class of Tetraterpenes. These are terpene molecules containing 10 consecutively linked isoprene units. (inferred from compound structure) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C50H72O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 705.1055 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 704.553231548 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | FGYPGICSXJEKCG-AENDIINCSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C50H72O2/c1-38(2)19-12-20-39(3)21-13-22-40(4)23-14-24-41(5)25-15-26-42(6)27-16-28-43(7)29-17-30-44(8)31-18-32-45(9)35-36-46-37-49(51)47-33-10-11-34-48(47)50(46)52/h10-11,19,21,23,25,27,29,31,33-35,37,51-52H,12-18,20,22,24,26,28,30,32,36H2,1-9H3/b39-21+,40-23+,41-25+,42-27+,43-29+,44-31+,45-35+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]naphthalene-1,4-diol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 2-demethylmenaquinol-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC1=C(O)C2=CC=CC=C2C(O)=C1)=C(\C)CCC=C(C)C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as polyprenyl quinols. These are compounds containing a polyisoprene chain attached to a quinol(hydroquinone) at the second ring position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Quinone and hydroquinone lipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Polyprenyl quinols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homopolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2-Demethylmenaquinone 8 + 2 Hydrogen ion + Hydrogen (gas) > 2-Demethylmenaquinol 8 +2 Hydrogen ion 2-Demethylmenaquinol 8 + Hydrogen ion + Trimethylamine N-Oxide > 2-Demethylmenaquinone 8 + Water + Trimethylamine 2-Demethylmenaquinol 8 + Dimethyl sulfoxide > 2-Demethylmenaquinone 8 + Dimethyl sulfide + Water 2-Demethylmenaquinone 8 + Glycerol 3-phosphate > 2-Demethylmenaquinol 8 + Dihydroxyacetone phosphate 2-Demethylmenaquinone 8 + 4 Hydrogen ion + NADH > 2-Demethylmenaquinol 8 + NAD +3 Hydrogen ion 2-Demethylmenaquinone 8 + Glycolic acid > 2-Demethylmenaquinol 8 + Glyoxylic acid 2-Demethylmenaquinol 8 + Fumaric acid > 2-Demethylmenaquinone 8 + Succinic acid 2-Demethylmenaquinone 8 + Hydrogen ion + NADH > 2-Demethylmenaquinol 8 + NAD 2-Demethylmenaquinone 8 + Hydrogen ion + NADPH > 2-Demethylmenaquinol 8 + NADP 2-Demethylmenaquinol 8 + S-Adenosylmethionine > S-Adenosylhomocysteine + Hydrogen ion + Menaquinol 8 1,4-Dihydroxy-2-naphthoic acid + Hydrogen ion + Octaprenyl diphosphate > 2-Demethylmenaquinol 8 + Carbon dioxide + Pyrophosphate all-<i>trans</i>-octaprenyl diphosphate + 1,4-Dihydroxy-2-naphthoic acid + Hydrogen ion > 2-Demethylmenaquinol 8 + Pyrophosphate + Carbon dioxide 2-Demethylmenaquinol 8 + S-adenosyl-L-methionine > Hydrogen ion + S-Adenosylhomocysteine + Menaquinol 8 1,4-dihydroxy-2-naphthoate + Hydrogen ion + Farnesylfarnesylgeranyl-PP + 1,4-Dihydroxy-2-naphthoyl-CoA > Carbon dioxide + Pyrophosphate + 2-Demethylmenaquinol 8 Octaprenyl diphosphate + 1,4-dihydroxy-2-naphthoate + Hydrogen ion + Octaprenyl diphosphate + 1,4-Dihydroxy-2-naphthoyl-CoA > Carbon dioxide + Pyrophosphate + 2-Demethylmenaquinol 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||