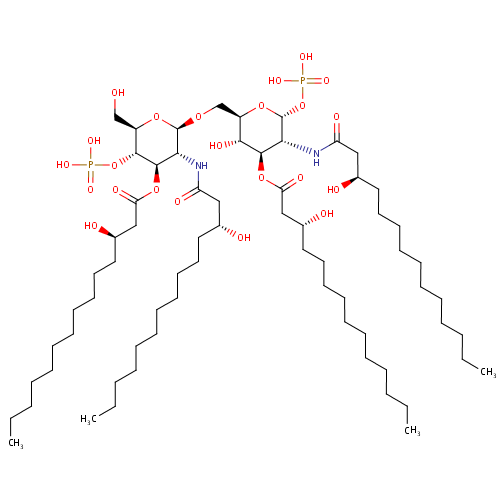
2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate (PAMDB001545)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001545 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2,3,2'3'-tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate is a member of the chemical class known as Dihexoses. These are disaccharides containing two hexose carbohydrates. Lipid IV(A) is involved in KDO metabolism. The enzyme is a single polypeptide that catalyzes the transfer of two KDO residues to a tetraacyldisaccharide-1,4'-bisphosphate precursor of lipid A, designated lipid IVA (Belunis, C. (PMID 7499229) Pseudomonas aeruginosa KdtA (EcKdtA) is a bifunctional enzyme that transfers two KDO units from two CMP-KDO molecules to lipid IV(A). (PMID 20394418) The analog, KDO-lipid IVA, functions as an acceptor, but is mannosylated at less than 1% the rate of KDO2-lipid IVA. (PMID 9446588) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C68H130N2O23P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 1405.7069 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 1404.8539615 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | KVJWZTLXIROHIL-QDORLFPLSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C68H130N2O23P2/c1-5-9-13-17-21-25-29-33-37-41-51(72)45-57(76)69-61-65(90-59(78)47-53(74)43-39-35-31-27-23-19-15-11-7-3)63(80)56(89-68(61)93-95(84,85)86)50-87-67-62(70-58(77)46-52(73)42-38-34-30-26-22-18-14-10-6-2)66(64(55(49-71)88-67)92-94(81,82)83)91-60(79)48-54(75)44-40-36-32-28-24-20-16-12-8-4/h51-56,61-68,71-75,80H,5-50H2,1-4H3,(H,69,76)(H,70,77)(H2,81,82,83)(H2,84,85,86)/t51-,52-,53-,54-,55-,56-,61-,62-,63-,64-,65-,66-,67-,68-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3R,4R,5S,6R)-5-hydroxy-6-({[(2R,3R,4R,5S,6R)-6-(hydroxymethyl)-3-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-5-(phosphonooxy)oxan-2-yl]oxy}methyl)-3-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-2-yl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | lipid iva | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CCCCCCCCCCC[C@@H](O)CC(=O)N[C@H]1[C@@H](OP(O)(O)=O)O[C@H](CO[C@@H]2O[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@H]2NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](O)[C@@H]1OC(=O)C[C@H](O)CCCCCCCCCCC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as saccharolipids. These are compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a sugar substitutes for the glycerol backbone that is present in glycerolipids and glycerophospholipids. The most familiar saccharolipids contain an acylated glucosamine. In contrast to others glycolipids, the fatty acid is not glycosidically linked to the sugar moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Saccharolipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Saccharolipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + 2,3,2',3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1-phosphate > ADP + Hydrogen ion + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate CMP-3-Deoxy-D-manno-octulosonate + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate > Cytidine monophosphate + Hydrogen ion + KDO-lipid IV(A) 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate + CMP-3-Deoxy-D-manno-octulosonate + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate <> KDO-lipid IV(A) + Cytidine monophosphate 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate + CMP-3-Deoxy-D-manno-octulosonate > alpha-Kdo-(2->6)-lipid IV(A) + Cytidine monophosphate undecaprenyl phosphate-4-amino-4-deoxy-L-arabinose + KDO2-Lipid A + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate <> alpha-Kdo-(2->4)-alpha-Kdo-(2->6)-[4-P-L-Ara4N]-lipid A + Di-trans,poly-cis-undecaprenyl phosphate + Lipid IIA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||