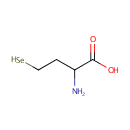
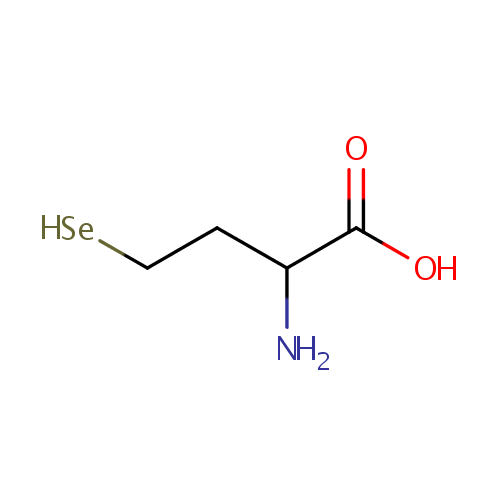
| References: |
- Esaki N, Nakamura T, Tanaka H, Suzuki T, Morino Y, Soda K: Enzymatic synthesis of selenocysteine in rat liver. Biochemistry. 1981 Jul 21;20(15):4492-6. Pubmed: 6456763
- Esaki N, Seraneeprakarn V, Tanaka H, Soda K: Purification and characterization of Clostridium sticklandii D-selenocystine alpha, beta-lyase. J Bacteriol. 1988 Feb;170(2):751-6. Pubmed: 3338973
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Liu G, Nellaiappan K, Kagan HM: Irreversible inhibition of lysyl oxidase by homocysteine thiolactone and its selenium and oxygen analogues. Implications for homocystinuria. J Biol Chem. 1997 Dec 19;272(51):32370-7. Pubmed: 9405445
- Soda, K., Oikawa, T., Esaki, N. (1999). "Vitamin B6 enzymes participating in selenium amino acid metabolism." Biofactors 10:257-262. Pubmed: 10609891
|
|---|