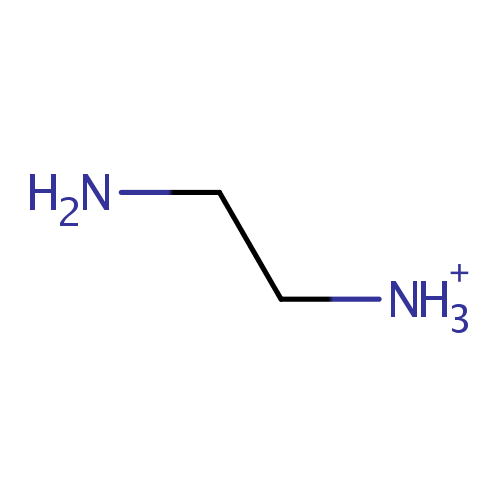
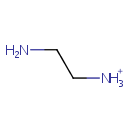Ethylenediamine (PAMDB001114)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001114 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Ethylenediamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a strongly basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000,000 kg being produced in 1998.In terms of quantities produced, ethylenediamine is the most significant diamine (aside from diaminohexane, which is a precursor to Nylon 6-6). Related derivatives of ethylenediamine include tetramethylethylenediamine, abbreviated (TMEDA), (CH3)2N-CH2CH2-N(CH3)2 and tetraethylethylenediamine, abbreviated (TEEDA), (C2H5)2N-CH2CH2-N(C2H5)2The bleaching activator tetraacetylethylenediamine is generated from ethylenediamine. The derivative N,N-ethylenebis(stearamide) (EBS) is a commercially significant mold-release agent and a surfactant in gasoline and motor oil." | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C2H9N2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 61.1063 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 61.076573298 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PIICEJLVQHRZGT-UHFFFAOYSA-O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C2H8N2/c3-1-2-4/h1-4H2/p+1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 333-18-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-aminoethan-1-aminium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 2-aminoethanaminium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NCC[NH3+] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as quaternary ammonium salts. These are compounds containing positively charged polyatomic ion of the structure NR4+, R being an alkyl group or an aryl group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organonitrogen compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Quaternary ammonium salts | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Quaternary ammonium salts | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 9 ?C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Hydrogen ion + Ornithine + L-Ornithine <> Carbon dioxide + Putrescine + Ethylenediamine S-Adenosylmethioninamine + Putrescine + Ethylenediamine <> 5'-Methylthioadenosine + Hydrogen ion + Spermidine Adenosine triphosphate + L-Glutamate + Putrescine + Ethylenediamine <> ADP + gamma-Glutamyl-L-putrescine + Hydrogen ion + Phosphate Agmatine + Water <> Putrescine + Urea + Ethylenediamine Ethylenediamine + alpha-Ketoglutarate + 4-Aminobutyraldehyde <> 1-Pyrroline + L-Glutamate + Water | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||