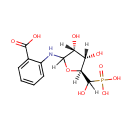
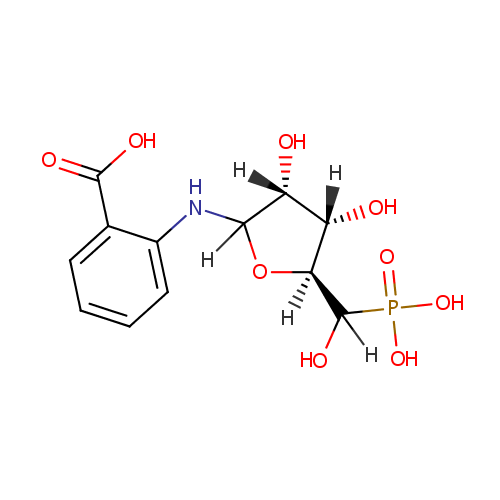
| InChI: | InChI=1S/C12H16NO9P/c14-7-8(15)10(22-9(7)12(18)23(19,20)21)13-6-4-2-1-3-5(6)11(16)17/h1-4,7-10,12-15,18H,(H,16,17)(H2,19,20,21)/t7-,8+,9-,10?,12?/m0/s1 |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Priestle, J. P., Grutter, M. G., White, J. L., Vincent, M. G., Kania, M., Wilson, E., Jardetzky, T. S., Kirschner, K., Jansonius, J. N. (1987). "Three-dimensional structure of the bifunctional enzyme N-(5'-phosphoribosyl)anthranilate isomerase-indole-3-glycerol-phosphate synthase from Escherichia coli." Proc Natl Acad Sci U S A 84:5690-5694. Pubmed: 3303031
- Sterner, R., Kleemann, G. R., Szadkowski, H., Lustig, A., Hennig, M., Kirschner, K. (1996). "Phosphoribosyl anthranilate isomerase from Thermotoga maritima is an extremely stable and active homodimer." Protein Sci 5:2000-2008. Pubmed: 8897600
|
|---|