|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000955 |
|---|
|
Identification |
|---|
| Name: |
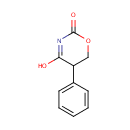
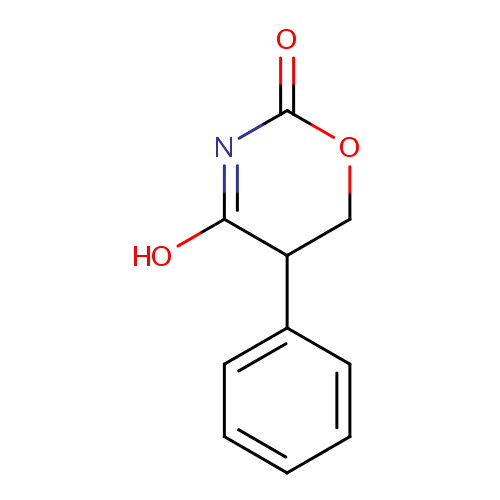
5-Phenyl-1,3-oxazinane-2,4-dione |
|---|
| Description: | 5-phenyl-1,3-oxazinane-2,4-dione belongs to the class of Aromatic Homomonocyclic Compounds. These are aromatic compounds containig only one ring, which is homocyclic. (inferred from compound structure) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3,3'-dihydroxy-β,β-carotene-4,4'-dione
- 3,3'-dihydroxy-4,4'-diketo-β,β-carotene
- 5-phenyl-1,3-oxazinane-2,4-dione
|
|---|
|
Chemical Formula: |
C10H9NO3 |
|---|
| Average Molecular Weight: |
191.1834 |
|---|
| Monoisotopic Molecular
Weight: |
191.058243159 |
|---|
| InChI Key: |
YPIQXPKZXKWWSZ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H9NO3/c12-9-8(6-14-10(13)11-9)7-4-2-1-3-5-7/h1-5,8H,6H2,(H,11,12,13) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 4-hydroxy-5-phenyl-5,6-dihydro-2H-1,3-oxazin-2-one |
|---|
|
Traditional IUPAC Name: |
4-hydroxy-5-phenyl-5,6-dihydro-1,3-oxazin-2-one |
|---|
| SMILES: | OC1=NC(=O)OCC1C1=CC=CC=C1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Benzene and substituted derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monocyclic benzene moiety
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|