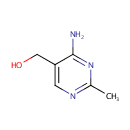
4-Amino-5-hydroxymethyl-2-methylpyrimidine (PAMDB000934)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000934 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 4-Amino-5-hydroxymethyl-2-methylpyrimidine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 4-amino-5-hydroxymethyl-2-methylpyrimidine is a member of the chemical class known as Pyrimidines and Pyrimidine Derivatives. These are compounds containing a pyrimidne ring, which is a six-member aromatic heterocycle which consists of two nitrogen atoms (at positions 1 and 3) and four carbon atoms. The conversion of 5-aminoimidazole ribonucleotide (AIR) into 4-amino-2-methyl-5-hydroxymethylpyrimidine (HMP) is a fascinating reaction on the thiamin biosynthetic pathway in bacteria and is probably the most complex unresolved rearrangement in primary metabolism. (PMID 15326535) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H9N3O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 139.1552 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 139.074561925 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | VUTBELPREDJDDH-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H9N3O/c1-4-8-2-5(3-10)6(7)9-4/h2,10H,3H2,1H3,(H2,7,8,9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 73-67-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (4-amino-2-methylpyrimidin-5-yl)methanol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | toxopyrimidine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=NC=C(CO)C(N)=N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as aminopyrimidines and derivatives. These are organic compounds containing an amino group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Diazines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidines and pyrimidine derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Aminopyrimidines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 198 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 4-Amino-5-hydroxymethyl-2-methylpyrimidine + Adenosine triphosphate <> 4-Amino-2-methyl-5-phosphomethylpyrimidine + ADP + Hydrogen ion + 4-amino-5-phosphonooxymethyl-2-methylpyrimidine Adenosine triphosphate + 4-Amino-5-hydroxymethyl-2-methylpyrimidine <> ADP + 4-Amino-2-methyl-5-phosphomethylpyrimidine 4-Amino-5-hydroxymethyl-2-methylpyrimidine + S-Adenosylmethionine <> 5-Aminoimidazole ribonucleotide + 4-Amino-2-methyl-5-phosphomethylpyrimidine + 5'-Deoxyadenosine + L-Methionine + Formic acid + CO Adenosine triphosphate + 4-Amino-5-hydroxymethyl-2-methylpyrimidine <> Hydrogen ion + ADP + 4-Amino-2-methyl-5-phosphomethylpyrimidine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||