|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000929 |
|---|
|
Identification |
|---|
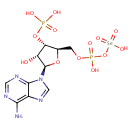
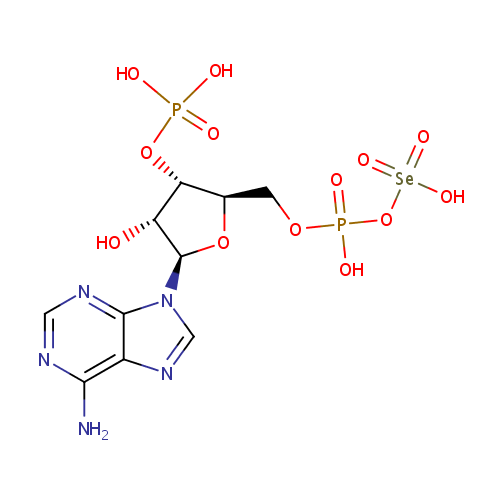
| Name: |
3'-Phosphoadenylylselenate |
|---|
| Description: | 3'-phosphoadenylylselenate is a member of the chemical class known as Purine Ribonucleoside 3',5'-Bisphosphates. These are purine ribobucleotides with one phosphate group attached to 3' and 5' hydroxyl groups of the ribose moiety. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3'-Phosphoadenosine-5'-phosphoselanate
- 3'-Phosphoadenosine-5'-phosphoselanic acid
- 3'-Phosphoadenylylselenic acid
|
|---|
|
Chemical Formula: |
C10H15N5O13P2Se |
|---|
| Average Molecular Weight: |
554.16 |
|---|
| Monoisotopic Molecular
Weight: |
554.930680443 |
|---|
| InChI Key: |
AZRLZPIFEZUZLW-KQYNXXCUSA-N |
|---|
| InChI: | InChI=1S/C10H15N5O13P2Se/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(27-29(17,18)19)4(26-10)1-25-30(20,21)28-31(22,23)24/h2-4,6-7,10,16H,1H2,(H,20,21)(H2,11,12,13)(H2,17,18,19)(H,22,23,24)/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]selenonic acid |
|---|
|
Traditional IUPAC Name: |
3'-phosphoadenylylselenate |
|---|
| SMILES: | NC1=C2N=CN([C@@H]3O[C@H](COP(O)(=O)O[Se](O)(=O)=O)[C@@H](OP(O)(O)=O)[C@H]3O)C2=NC=N1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside 3',5'-bisphosphates. These are purine ribobucleotides with one phosphate group attached to 3' and 5' hydroxyl groups of the ribose moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside 3',5'-bisphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside monophosphate
- N-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic selenate
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- Monosaccharide
- Saccharide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|