|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000808 |
|---|
|
Identification |
|---|
| Name: |
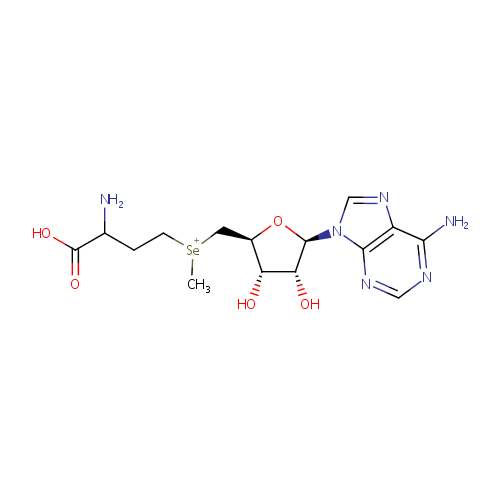
Se-Adenosylselenomethionine |
|---|
| Description: | Se-Adenosylselenomethionine is an intermediate in Selenoamino acid metabolism. Se-Adenosylselenomethionine is converted from Selenomethionine via the enzyme S-adenosylmethionine synthetase (EC 2.5.1.6). It is then |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C15H23N6O5Se |
|---|
| Average Molecular Weight: |
446.34 |
|---|
| Monoisotopic Molecular
Weight: |
447.089514704 |
|---|
| InChI Key: |
GGJFWMOVUFBSIN-YDBXVIPQSA-O |
|---|
| InChI: | InChI=1S/C15H22N6O5Se/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (3-amino-3-carboxypropyl)({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl})methylselanium |
|---|
|
Traditional IUPAC Name: |
se-adenosylselenomethionine |
|---|
| SMILES: | C[Se+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as glycosylamines. These are compounds consisting of an?amine?with a?beta-N-glycosidic bond?to a carbohydrate, thus forming a cyclic?hemiaminal ether?bond (alpha-amino ether). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Glycosyl compounds |
|---|
|
Direct Parent |
Glycosylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-glycosyl compound
- Alpha-amino acid or derivatives
- Alpha-amino acid
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Aminopyrimidine
- Amino fatty acid
- Fatty acyl
- Imidolactam
- Pyrimidine
- Primary aromatic amine
- N-substituted imidazole
- Monosaccharide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Selenoether
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Primary amine
- Organoselenium compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Alcohol
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB11118 | | Pubchem Compound ID | 24892761 | | Kegg ID | C05691 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|