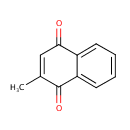
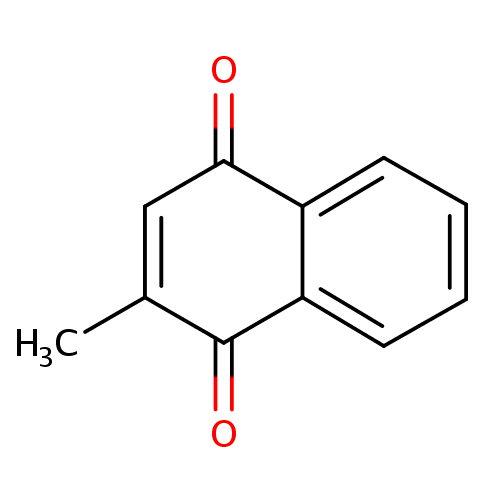
| References: |
- Chladek J, Martinkova J, Sispera L: An in vitro study on methotrexate hydroxylation in rat and human liver. Physiol Res. 1997;46(5):371-9. Pubmed: 9728483
- Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S: Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004 Jul 21;292(3):358-61. Pubmed: 15265851
- Harrington DJ, Soper R, Edwards C, Savidge GF, Hodges SJ, Shearer MJ: Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J Lipid Res. 2005 May;46(5):1053-60. Epub 2005 Feb 1. Pubmed: 15722567
- Iioka H, Moriyama IS, Morimoto K, Akada S, Hisanaga H, Ishihara Y, Ichijo M: Pharmacokinetics of vitamin K in mothers and children in the perinatal period: transplacental transport of vitamin K2 (MK-4). Asia Oceania J Obstet Gynaecol. 1991 Mar;17(1):97-100. Pubmed: 2064595
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kwasnicka-Crawford DA, Vincent SR: Role of a novel dual flavin reductase (NR1) and an associated histidine triad protein (DCS-1) in menadione-induced cytotoxicity. Biochem Biophys Res Commun. 2005 Oct 21;336(2):565-71. Pubmed: 16140270
- Lasalvia-Prisco E, Cucchi S, Vazquez J, Lasalvia-Galante E, Golomar W, Gordon W: Serum markers variation consistent with autoschizis induced by ascorbic acid-menadione in patients with prostate cancer. Med Oncol. 2003;20(1):45-52. Pubmed: 12665684
- Ono M, Ohta H, Ohhira M, Sekiya C, Namiki M: Measurement of immunoreactive prothrombin, des-gamma-carboxy prothrombin, and vitamin K in human liver tissues: overproduction of immunoreactive prothrombin in hepatocellular carcinoma. Am J Gastroenterol. 1990 Sep;85(9):1149-54. Pubmed: 1697141
- Price RJ, Mistry H, Wield PT, Renwick AB, Beamand JA, Lake BG: Comparison of the toxicity of allyl alcohol, coumarin and menadione in precision-cut rat, guinea-pig, cynomolgus monkey and human liver slices. Arch Toxicol. 1996;71(1-2):107-11. Pubmed: 9010592
- Robertson DG, Bailey DL, Martin RA: Species differences in response to photohemolytic agents. Photochem Photobiol. 1991 Apr;53(4):455-61. Pubmed: 1857739
- Snider BJ, Moss JL, Revilla FJ, Lee CS, Wheeler VC, Macdonald ME, Choi DW: Neocortical neurons cultured from mice with expanded CAG repeats in the huntingtin gene: unaltered vulnerability to excitotoxins and other insults. Neuroscience. 2003;120(3):617-25. Pubmed: 12895502
- Strunnikova N, Hilmer S, Flippin J, Robinson M, Hoffman E, Csaky KG: Differences in gene expression profiles in dermal fibroblasts from control and patients with age-related macular degeneration elicited by oxidative injury. Free Radic Biol Med. 2005 Sep 15;39(6):781-96. Pubmed: 16109308
- Suzuki S, Iwata G, Sutor AH: Vitamin K deficiency during the perinatal and infantile period. Semin Thromb Hemost. 2001;27(2):93-8. Pubmed: 11372776
- Thijssen HH, Drittij-Reijnders MJ: Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996 Jan;75(1):121-7. Pubmed: 8785182
- Usui Y, Tanimura H, Nishimura N, Kobayashi N, Okanoue T, Ozawa K: Vitamin K concentrations in the plasma and liver of surgical patients. Am J Clin Nutr. 1990 May;51(5):846-52. Pubmed: 2333843
- Wermuth B, Platts KL, Seidel A, Oesch F: Carbonyl reductase provides the enzymatic basis of quinone detoxication in man. Biochem Pharmacol. 1986 Apr 15;35(8):1277-82. Pubmed: 3083821
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|