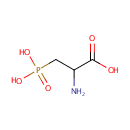
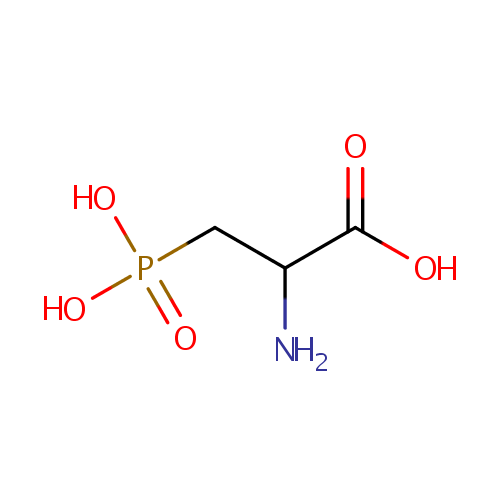
| References: |
- Acquavella JF, Alexander BH, Mandel JS, Burns CJ, Gustin C: Exposure misclassification in studies of agricultural pesticides: insights from biomonitoring. Epidemiology. 2006 Jan;17(1):69-74. Pubmed: 16357597
- Chan P, Mahler J: NTP technical report on the toxicity studies of Glyphosate (CAS No. 1071-83-6) Administered In Dosed Feed To F344/N Rats And B6C3F1 Mice. Toxic Rep Ser. 1992 Jul;16:1-D3. Pubmed: 12209170
- Dickson SJ, Meinhold RH, Beer ID, Koelmeyer TD: Rapid determination of glyphosate in postmortem specimens using 31P NMR. J Anal Toxicol. 1988 Sep-Oct;12(5):284-6. Pubmed: 3226127
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kintzios S, Pistola E, Panagiotopoulos P, Bomsel M, Alexandropoulos N, Bem F, Ekonomou G, Biselis J, Levin R: Bioelectric recognition assay (BERA). Biosens Bioelectron. 2001 Jun;16(4-5):325-36. Pubmed: 11390221
- Lee RK, Wurtman RJ, Cox AJ, Nitsch RM: Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8083-7. Pubmed: 7644542
- Lueken A, Juhl-Strauss U, Krieger G, Witte I: Synergistic DNA damage by oxidative stress (induced by H2O2) and nongenotoxic environmental chemicals in human fibroblasts. Toxicol Lett. 2004 Feb 28;147(1):35-43. Pubmed: 14700526
- Sorrentino G, Singh IN, Massarelli R, Kanfer JN: Stimulation of phospholipase C activity by norepinephrine, t-ACPD and bombesin in LA-N-2 cells. Eur J Pharmacol. 1996 Jul 11;308(1):81-6. Pubmed: 8836635
- Tan SA, Tan LG: Distribution of ciliatine (2-aminoethylphosphonic acid) and phosphonoalanine (2-amino-3-phosphonopropionic acid) in human tissues. Clin Physiol Biochem. 1989;7(6):303-9. Pubmed: 2627760
- Thompson JA, Miles BS, Fennessey PV: Urinary organic acids quantitated by age groups in a healthy pediatric population. Clin Chem. 1977 Sep;23(9):1734-8. Pubmed: 890917
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Wester RC, Melendres J, Sarason R, McMaster J, Maibach HI: Glyphosate skin binding, absorption, residual tissue distribution, and skin decontamination. Fundam Appl Toxicol. 1991 May;16(4):725-32. Pubmed: 1884912
|
|---|