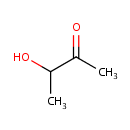
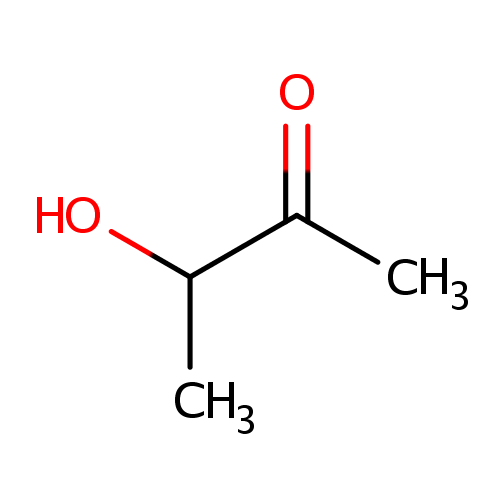
| References: |
- Bertagnolli, B. L., Hager, L. P. (1993). "Role of flavin in acetoin production by two bacterial pyruvate oxidases." Arch Biochem Biophys 300:364-371. Pubmed: 8424670
- Hirooka EY, Muller EE, Freitas JC, Vicente E, Yoshimoto Y, Bergdoll MS: Enterotoxigenicity of Staphylococcus intermedius of canine origin. Int J Food Microbiol. 1988 Dec;7(3):185-91. Pubmed: 3275321
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Otsuka M, Ohmori S: Simple and sensitive determination of diacetyl and acetoin in biological samples and alcoholic drinks by gas chromatography with electron-capture detection. J Chromatogr. 1992 Jun 10;577(2):215-20. Pubmed: 1400754
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Walker V, Mills GA, Hall MA, Lowes JA: Carbohydrate fermentation by gut microflora in preterm neonates. Arch Dis Child. 1989 Oct;64(10 Spec No):1367-73. Pubmed: 2589871
- Walker V, Mills GA: Urinary organic acid excretion by babies born before 33 weeks of gestation. Clin Chem. 1989 Jul;35(7):1460-6. Pubmed: 2758593
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|