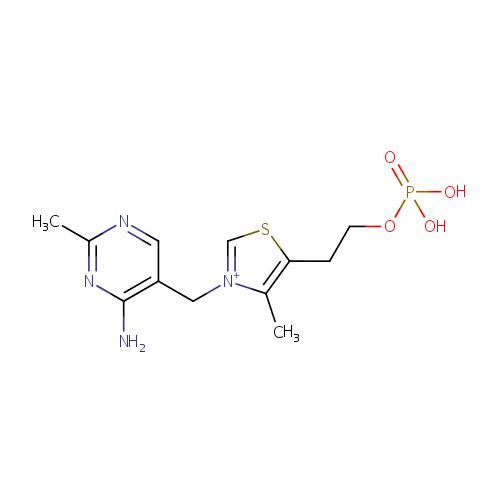
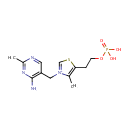Thiamine monophosphate (PAMDB000462)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000462 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Thiamine monophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Thiamine dihydrogen phosphate ester. The monophosphate ester of thiamine. Synonyms: monophosphothiamine; vitamin B1 monophosphate. -- Pubchem | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C12H17N4O4PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 344.327 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 344.070812254 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | HZSAJDVWZRBGIF-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C12H17N4O4PS/c1-8-11(3-4-20-21(17,18)19)22-7-16(8)6-10-5-14-9(2)15-12(10)13/h5,7H,3-4,6H2,1-2H3,(H3-,13,14,15,17,18,19) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 495-23-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-5-[2-(phosphonooxy)ethyl]-1,3-thiazol-3-ium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | thiamin monophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=C(CCO[P@](O)([O-])=O)SC=[N+]1CC1=CN=C(C)N=C1N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Diazines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidines and pyrimidine derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Thiamine phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Thiamine monophosphate <> ADP + Thiamine pyrophosphate Adenosine triphosphate + Thiamine <> ADP + Hydrogen ion + Thiamine monophosphate Water + Thiamine pyrophosphate > Hydrogen ion + Phosphate + Thiamine monophosphate 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + 4-Methyl-5-(2-phosphoethyl)-thiazole + Hydrogen ion <> Pyrophosphate + Thiamine monophosphate Adenosine triphosphate + Thiamine <> ADP + Thiamine monophosphate 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + 4-Methyl-5-(2-phosphoethyl)-thiazole <> Pyrophosphate + Thiamine monophosphate 2-[(2<i>R</i>,5<i>Z</i>)-(2-carboxy-4-methylthiazol-5(2<i>H</i>)-ylidene]ethyl phosphate + 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + Hydrogen ion > Thiamine monophosphate + Carbon dioxide + Pyrophosphate 4-amino-2-methyl-5-diphosphomethylpyrimidine + 2-((2R,5Z)-2-Carboxy-4-methylthiazol-5(2H)-ylidene)ethyl phosphate + 2 Hydrogen ion + 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate > Thiamine monophosphate + Carbon dioxide + diphosphate + Thiamine monophosphate + Pyrophosphate Thiamine monophosphate + Adenosine triphosphate + Thiamine monophosphate > Thiamine pyrophosphate + Adenosine diphosphate + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Leder, Irwin G. Enzymic synthesis of thiamine monophosphate. Journal of Biological Chemistry (1961), 236 3066-71. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||