|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000455 |
|---|
|
Identification |
|---|
| Name: |
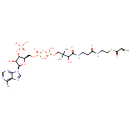
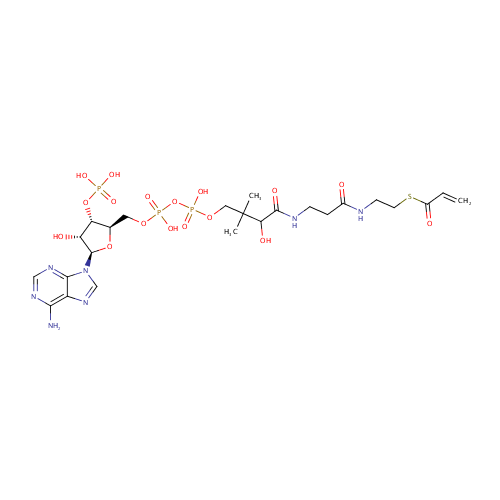
Acrylyl-CoA |
|---|
| Description: | Acrylyl-CoA is involved in alternative pathways of propionate metabolism. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Acryloyl coenzyme A
- Acryloyl-CoA
- Acryloyl-Coenzyme A
- Acrylyl coenzyme A
- Acrylyl-CoA
- Acrylyl-Coenzyme A
- CoA S-2-propenoate
- CoA S-2-propenoic acid
- CoA S-acrylate
- CoA S-acrylic acid
- Coenzyme A S-2-propenoate
- Coenzyme A S-2-propenoic acid
- Coenzyme A S-acrylate
- Coenzyme A S-acrylic acid
- Propenoyl-coa
- Thioacrylate S-ester with coenzyme A
- Thioacrylic acid S-ester with coenzyme A
|
|---|
|
Chemical Formula: |
C24H38N7O17P3S |
|---|
| Average Molecular Weight: |
821.582 |
|---|
| Monoisotopic Molecular
Weight: |
821.125773051 |
|---|
| InChI Key: |
POODSGUMUCVRTR-UXYNFSPESA-N |
|---|
| InChI: | InChI=1S/C24H38N7O17P3S/c1-4-15(33)52-8-7-26-14(32)5-6-27-22(36)19(35)24(2,3)10-45-51(42,43)48-50(40,41)44-9-13-18(47-49(37,38)39)17(34)23(46-13)31-12-30-16-20(25)28-11-29-21(16)31/h4,11-13,17-19,23,34-35H,1,5-10H2,2-3H3,(H,26,32)(H,27,36)(H,40,41)(H,42,43)(H2,25,28,29)(H2,37,38,39)/t13-,17-,18-,19?,23-/m1/s1 |
|---|
| CAS
number: |
5776-58-9 |
|---|
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-2,2-dimethyl-3-[(2-{[2-(prop-2-enoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-[({hydroxy[hydroxy(3-hydroxy-2,2-dimethyl-3-[(2-{[2-(prop-2-enoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C(N)N=CN=C12)C(O)C(=O)NCCC(=O)NCCSC(=O)C=C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- N-glycosyl compound
- Glycosyl compound
- Beta amino acid or derivatives
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- N-acyl-amine
- Monosaccharide
- Fatty amide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Thiocarboxylic acid ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Thioether
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid amide
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Microbial metabolism in diverse environments pae01120
- Propanoate metabolism pae00640
- Reductive carboxylate cycle (CO2 fixation) pae00720
- beta-Alanine metabolism pae00410
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- OMMBID: http://genetics.accessmedicine.com/server-java/Arknoid/amed/mmbid/co_chapters/ch094/ch094_p03.html: http://genetics.accessmedicine.com/server-java/Arknoid/amed/mmbid/co_chapters/ch094/ch094_p03.html
|
|---|
| Synthesis Reference: |
Symes, Kenneth Charles; Collier, Simon Andrew; Armitage, Yvonne Christine; Mistry, Rajesh; Baranyai, Robert. Biocatalytic manufacturing of (meth)acrylic esters. PCT Int. Appl. (2007), 40pp. CODEN: PIXXD2 WO 2007039415 A1 20070412 CAN 146:40 |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|