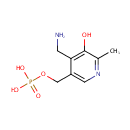
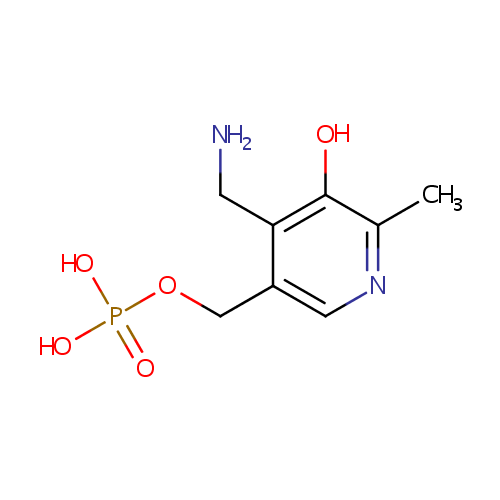
Pyridoxamine 5'-phosphate (PAMDB000420)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000420 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Pyridoxamine 5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Pyradoxamine 5' phosphate or Vitamin B6 is a water-soluble compound that was discovered in 1930s during nutrition studies on rats. The vitamin was named pyridoxine to indicate its structural homology to pyridine. Later it was shown that vitamin B6 could exist in two other, slightly different, chemical forms, termed pyridoxal and pyridoxamine. All three forms of vitamin B6 are precursors of an activated compound known as pyridoxal 5-phosphate (PLP), which plays a vital role as the cofactor of a large number of essential enzymes.Vitamin B6 is a water-soluble vitamin. The three major forms of vitamin B6 are pyridoxine (also known as pyridoxol), pyridoxal, and pyridoxamine, which are all converted in to pyridoxal 5-phosphate (PLP) a cofactor in many reactions of amino acid metabolism. PLP also is necessary for the enzymatic reaction governing the release of glucose from glycogen. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C8H13N2O5P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 248.173 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 248.056208048 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ZMJGSOSNSPKHNH-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C8H13N2O5P/c1-5-8(11)7(2-9)6(3-10-5)4-15-16(12,13)14/h3,11H,2,4,9H2,1H3,(H2,12,13,14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 529-96-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[4-(aminomethyl)-5-hydroxy-6-methylpyridin-3-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | pyridoxamine-5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=NC=C(COP(O)(O)=O)C(CN)=C1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyridoxamines. These are pyridoxal derivatives in which the pyridoxal moiety is substituted at position 2 by an amine. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyridoxamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyridoxamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Alanine + Pyridoxal 5'-phosphate > Pyridoxamine 5'-phosphate + Pyruvic acid D-Alanine + Pyridoxal 5'-phosphate > Pyridoxamine 5'-phosphate + Pyruvic acid Water + Oxygen + Pyridoxamine 5'-phosphate > Hydrogen peroxide + Ammonium + Pyridoxal 5'-phosphate Pyridoxamine 5'-phosphate + Water + Oxygen <> Pyridoxal 5'-phosphate + Ammonia + Hydrogen peroxide Adenosine triphosphate + Pyridoxamine <> ADP + Pyridoxamine 5'-phosphate Oxygen + Water + Pyridoxamine 5'-phosphate > Hydrogen ion + Hydrogen peroxide + Ammonia + Pyridoxal 5'-phosphate Adenosine triphosphate + Pyridoxamine <> Hydrogen ion + ADP + Pyridoxamine 5'-phosphate Pyridoxamine 5'-phosphate + Oxoglutaric acid <> Pyridoxal 5'-phosphate + D-Glutamic acid Pyridoxamine 5'-phosphate + Water + Oxygen + Pyridoxine 5'-phosphate <> Pyridoxal 5'-phosphate + Ammonia + Hydrogen peroxide Pyridoxamine + Adenosine triphosphate > Pyridoxamine 5'-phosphate + Adenosine diphosphate + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Katsunishi, Masatoshi; Kondo, Osamu. Pyridoxamine-5'-phosphate. Jpn. Tokkyo Koho (1972), 2 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||