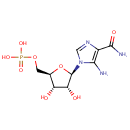
AICAR (PAMDB000407)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000407 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | AICAR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | AICAR (5-amino-4-carboxamide imidazole riboside 5'-phosphate), is a by-product of histidine biosynthesis, and its (ribo)triphosphate derivative, ZTP, has been detected in Pseudomonas aeruginosa. It is also an intermediate in the generation of inosine monophosphate. The purH gene product catalyzes the conversion of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) to 5-form-aminoimidazole-4-carboxamide ribonucleotide (FAICAR) and inosine. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H15N4O8P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 338.2112 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 338.062749988 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NOTGFIUVDGNKRI-UUOKFMHZSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H15N4O8P/c10-7-4(8(11)16)12-2-13(7)9-6(15)5(14)3(21-9)1-20-22(17,18)19/h2-3,5-6,9,14-15H,1,10H2,(H2,11,16)(H2,17,18,19)/t3-,5-,6-,9-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 3031-94-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | aica ribonucleotide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC(=O)C1=C(N)N(C=N1)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as 1-phosphoribosyl-imidazoles. These are organic compounds containing the imidazole ring linked to a ribose phosphate through a 1-2 bond. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Imidazole ribonucleosides and ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | 1-phosphoribosyl-imidazoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 1-phosphoribosyl-imidazoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | AICAR + Pyrophosphate <> 5-Amino-4-imidazolecarboxyamide + Phosphoribosyl pyrophosphate Phosphoribulosylformimino-AICAR-P + L-Glutamine <> D-Erythro-imidazole-glycerol-phosphate + AICAR + L-Glutamate SAICAR <> Fumaric acid + AICAR N10-Formyl-THF + AICAR <> Tetrahydrofolic acid + Phosphoribosyl formamidocarboxamide Phosphoribulosylformimino-AICAR-P + L-Glutamine > Hydrogen ion + L-Glutamate + D-Erythro-imidazole-glycerol-phosphate + AICAR Adenylsuccinic acid + SAICAR <> Fumaric acid + Adenosine monophosphate + AICAR Tetrahydrofolic acid + FAICAR + Tetrahydrofolic acid > 10-Formyltetrahydrofolate + AICAR + N10-Formyl-THF Phosphoribosyl-AMP + Water > AICAR AICAR > PhosphoribosylformiminoAICAR-phosphate SAICAR + SAICAR > AICAR + Fumaric acid AICAR + 10-Formyltetrahydrofolate + N10-Formyl-THF > FAICAR + tetrahydropteroyl mono-L-glutamate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Schmitt, Laurent; Caperelli, Carol A. Enantiospecific synthesis of carbocyclic aminoimidazole carboxamide ribonucleotide (C-AICAR), succinoaminoimidazole carboxamide ribonucleotide (C-SAICAR), and a new intermediate for SAICAR analogs. Nucleosides & N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||