|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000292 |
|---|
|
Identification |
|---|
| Name: |
Dyspropterin |
|---|
| Description: | Dyspropterin, an intermediate formed from dihydroneopterin triphosphate in the biosynthetic pathway of tetrahydrobiopterin. |
|---|
|
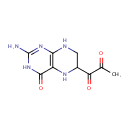
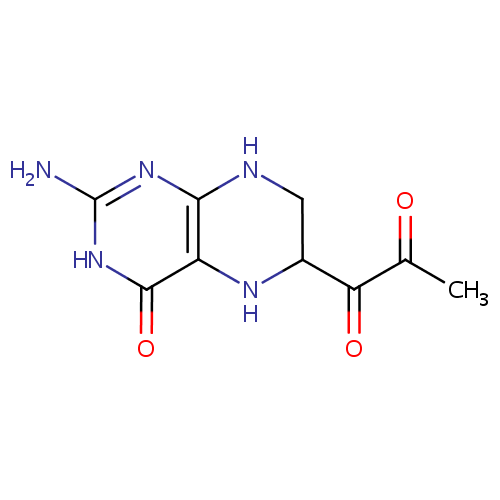
Structure |
|
|---|
| Synonyms: | - 6-(1,2-Dioxopropyl)-5,6,7,8-tetrahydropterin
- 6-Pyruvoyl-5,6,7,8-tetrahydropterin
- 6-Pyruvoyl-tetrahydropterin
- 6-Pyruvoyltetrahydropterin
- Dyspropterin
- PPH4
- Pyruvoyl-H4-pterin
- Pyruvoyl-H4-pterin
|
|---|
|
Chemical Formula: |
C9H11N5O3 |
|---|
| Average Molecular Weight: |
237.2153 |
|---|
| Monoisotopic Molecular
Weight: |
237.086189243 |
|---|
| InChI Key: |
WBJZXBUVECZHCE-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H11N5O3/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7/h4,12H,2H2,1H3,(H4,10,11,13,14,17) |
|---|
| CAS
number: |
89687-39-8 |
|---|
| IUPAC Name: | 1-(2-amino-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl)propane-1,2-dione |
|---|
|
Traditional IUPAC Name: |
1-(2-amino-4-oxo-5,6,7,8-tetrahydro-3H-pteridin-6-yl)propane-1,2-dione |
|---|
| SMILES: | CC(=O)C(=O)C1CNC2=C(N1)C(=O)NC(N)=N2 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pterins and derivatives. These are polycyclic aromatic compounds containing a pterin moiety, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
|
Direct Parent |
Pterins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pterin
- Secondary aliphatic/aromatic amine
- Pyrimidone
- Imidolactam
- Pyrimidine
- Primary aromatic amine
- Gamma-aminoketone
- Beta-aminoketone
- Alpha-diketone
- Heteroaromatic compound
- Vinylogous amide
- Alpha-aminoketone
- Lactam
- Ketone
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Bonafe L, Thony B, Leimbacher W, Kierat L, Blau N: Diagnosis of dopa-responsive dystonia and other tetrahydrobiopterin disorders by the study of biopterin metabolism in fibroblasts. Clin Chem. 2001 Mar;47(3):477-85. Pubmed: 11238300
- Dhondt JL, Hayte JM: [Screening of tetrahydrobiopterin deficiency among hyperphenylalaninemic patients] Ann Biol Clin (Paris). 2002 Mar-Apr;60(2):165-71. Pubmed: 11937441
- Iwanaga N, Yamamasu S, Tachibana D, Nishio J, Nakai Y, Shintaku H, Ishiko O: Activity of synthetic enzymes of tetrahydrobiopterin in the human placenta. Int J Mol Med. 2004 Jan;13(1):117-20. Pubmed: 14654981
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Niederwieser A, Curtius HC: Tetrahydrobiopterin biosynthetic pathway and deficiency. Enzyme. 1987;38(1-4):302-11. Pubmed: 3326735
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|