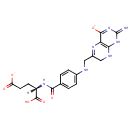
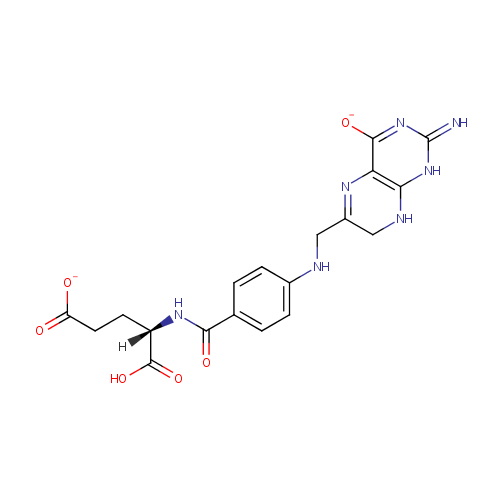
Dihydrofolic acid (PAMDB000235)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000235 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Dihydrofolic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Dihydrofolic acid is a folic acid derivative acted upon by dihydrofolate reductase to produce tetrahydrofolic acid. It interacts with bacteria during cell division. It can be targeted with drug analogs to prevent nucleic acid synthesis. Dihydrofolic acid is also known by the name Dihydrofolate - more commonly Vitamin B9. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C19H21N7O6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 443.4133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 443.155331439 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | OZRNSSUDZOLUSN-LBPRGKRZSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C19H21N7O6/c20-19-25-15-14(17(30)26-19)23-11(8-22-15)7-21-10-3-1-9(2-4-10)16(29)24-12(18(31)32)5-6-13(27)28/h1-4,12,21H,5-8H2,(H,24,29)(H,27,28)(H,31,32)(H4,20,22,25,26,30)/t12-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 4033-27-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (4S)-4-carboxy-4-[(4-{[(2-imino-4-oxido-1,2,7,8-tetrahydropteridin-6-yl)methyl]amino}phenyl)formamido]butanoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (4S)-4-carboxy-4-[(4-{[(2-imino-4-oxido-7,8-dihydro-1H-pteridin-6-yl)methyl]amino}phenyl)formamido]butanoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC2=C(N=C(CNC3=CC=C(C=C3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)CN2)C(=O)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Dihydrofolic acid + Hydrogen ion + NADPH <> NADP + Tetrahydrofolic acid dUMP + 5,10-Methylene-THF <> Dihydrofolic acid + 5-Thymidylic acid Tetrahydrofolic acid + NAD <> Dihydrofolic acid + NADH + Hydrogen ion Dihydrofolic acid + NAD <> Folic acid + NADH + Hydrogen ion Dihydrofolic acid + NADP <> Folic acid + NADPH + Hydrogen ion Adenosine triphosphate + 7,8-Dihydropteroic acid + L-Glutamate <> ADP + Phosphate + Dihydrofolic acid L-Glutamate + 7,8-Dihydropteroic acid + Adenosine triphosphate > Hydrogen ion + Dihydrofolic acid + Phosphate + ADP Tetrahydrofolic acid + NADP > Dihydrofolic acid + NADPH Adenosine triphosphate + 7,8-Dihydropteroic acid + L-Glutamate > ADP + Inorganic phosphate + Dihydrofolic acid Dihydrofolic acid + NADP + Dihydrofolic acid > Folic acid + NADPH + Hydrogen ion + NADPH Dihydrofolic acid + NADPH + Hydrogen ion + Dihydrofolic acid + NADPH > Tetrahydrofolic acid + NADP + Tetrahydrofolic acid 7,8-dihydrofolate monoglutamate + Hydrogen ion + NADPH + Dihydrofolic acid + NADPH > NADP + Tetrahydrofolic acid + Tetrahydrofolic acid Dihydrofolic acid + 5-Thymidylic acid + Dihydrofolic acid > 5,10-Methylene-THF + dUMP + 5,10-Methylene-THF dUMP + 5,10-methenyltetrahydrofolate mono-L-glutamate + 5,10-methenyltetrahydrofolate mono-L-glutamate > Dihydrofolic acid + 5-Thymidylic acid + Dihydrofolic acid 7,8-Dihydropteroic acid + Adenosine triphosphate + L-Glutamic acid + L-Glutamate > Adenosine diphosphate + Phosphate + Hydrogen ion + 7,8-dihydrofolate monoglutamate + ADP + Dihydrofolic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Smith, Karin; Scrimgeour, K. G.; Huennekens, F. M. Folic acid coenzymes and one-carbon metabolism. XV. Synthesis of a new form of dihydrofolate. Biochemical and Biophysical Research Communications (1963), 11(5), 388-92. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||