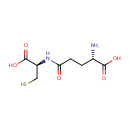
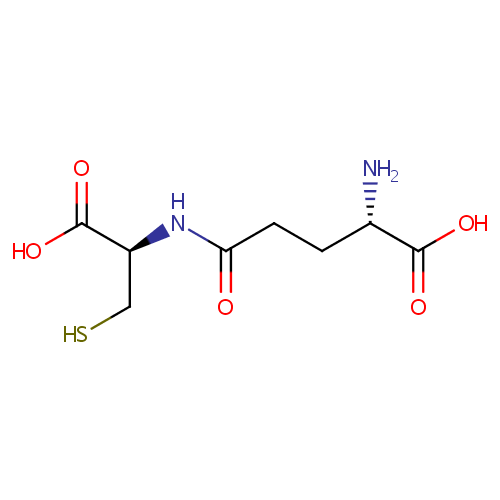
gamma-Glutamylcysteine (PAMDB000232)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000232 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | gamma-Glutamylcysteine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | G-Glutamylcysteine is a product of enzyme glutamate-cysteine ligase [EC 6.3.2.2] and a substrate of enzyme glutathione synthase [EC 6.3.2.3] in glutamate metabolism pathway (KEGG). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C8H14N2O5S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 250.272 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 250.062342258 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | RITKHVBHSGLULN-WHFBIAKZSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C8H14N2O5S/c9-4(7(12)13)1-2-6(11)10-5(3-16)8(14)15/h4-5,16H,1-3,9H2,(H,10,11)(H,12,13)(H,14,15)/t4-,5-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 636-58-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-carboxy-2-sulfanylethyl]carbamoyl}butanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | gamma-glutamylcysteine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | N[C@@H](CCC(=O)N[C@@H](CS)C(O)=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as gamma-glutamyl amino acids. These are dipeptides consisting of any C-terminal amino acid having a gamma-glutamyl residue attached at the N alpha-position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Gamma-glutamyl amino acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + L-Cysteine + L-Glutamate <> ADP + gamma-Glutamylcysteine + Hydrogen ion + Phosphate Adenosine triphosphate + gamma-Glutamylcysteine + Glycine <> ADP + Glutathione + Hydrogen ion + Phosphate Adenosine triphosphate + gamma-Glutamylcysteine + Glycine <> ADP + Phosphate + Glutathione Adenosine triphosphate + L-Glutamate + L-Cysteine <> ADP + Phosphate + gamma-Glutamylcysteine Adenosine triphosphate + L-Glutamate + L-Cysteine > ADP + Inorganic phosphate + gamma-Glutamylcysteine Adenosine triphosphate + gamma-Glutamylcysteine + Glycine > ADP + Inorganic phosphate + Glutathione L-Glutamic acid + Adenosine triphosphate + L-Cysteine + L-Glutamate > Adenosine diphosphate + Phosphate + Hydrogen ion + gamma-Glutamylcysteine + ADP gamma-Glutamylcysteine + Glycine + Adenosine triphosphate > Hydrogen ion + Phosphate + Adenosine diphosphate + Glutathione + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Bridge, Wallace John; Zarka, Martin Hani. Enzymic production of g-glutamylcysteine. PCT Int. Appl. (2006), 76pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||