|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000225 |
|---|
|
Identification |
|---|
| Name: |
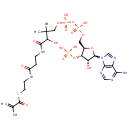
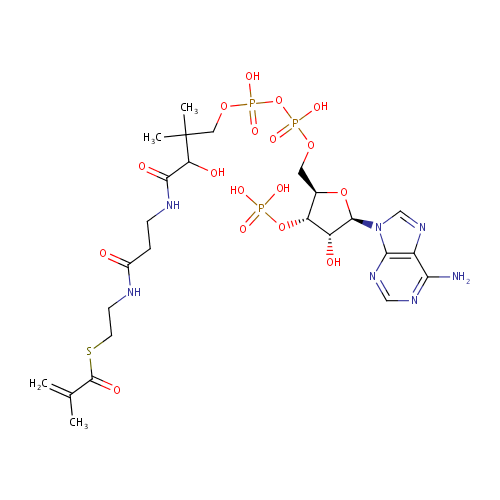
Methacrylyl-CoA |
|---|
| Description: | Methacrylyl-CoA is a metabolite in the valine, leucine and isoleucine degradation pathway and is highly reactive with free thiol compounds (PMID 14684172; KEGG). |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-Methylprop-2-enoyl-CoA
- 2-Methylprop-2-enoyl-Coenzyme A
- Methacrylyl CoA
- Methacrylyl Coenzyme A
- Methacrylyl-CoA
- Methacrylyl-coenzyme a
- Methylacrylyl-CoA
- Methylacrylyl-Coenzyme A
- S-(2-methyl-2-propenoate
- S-(2-Methyl-2-propenoate)
- S-(2-Methyl-2-propenoate) CoA
- S-(2-Methyl-2-propenoate) Coenzyme A
- S-(2-methyl-2-propenoic acid
- S-(2-Methyl-2-propenoic acid)
- S-(2-Methyl-2-propenoic acid) CoA
- S-(2-Methyl-2-propenoic acid) coenzyme A
|
|---|
|
Chemical Formula: |
C25H40N7O17P3S |
|---|
| Average Molecular Weight: |
835.608 |
|---|
| Monoisotopic Molecular
Weight: |
835.141423115 |
|---|
| InChI Key: |
NPALUEYCDZWBOV-NNYIDDMCSA-N |
|---|
| InChI: | InChI=1S/C25H40N7O17P3S/c1-13(2)24(37)53-8-7-27-15(33)5-6-28-22(36)19(35)25(3,4)10-46-52(43,44)49-51(41,42)45-9-14-18(48-50(38,39)40)17(34)23(47-14)32-12-31-16-20(26)29-11-30-21(16)32/h11-12,14,17-19,23,34-35H,1,5-10H2,2-4H3,(H,27,33)(H,28,36)(H,41,42)(H,43,44)(H2,26,29,30)(H2,38,39,40)/t14-,17-,18-,19?,23-/m1/s1 |
|---|
| CAS
number: |
6008-91-9 |
|---|
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy(3-hydroxy-2,2-dimethyl-3-{[2-({2-[(2-methylprop-2-enoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy)phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-[({hydroxy[hydroxy(3-hydroxy-2,2-dimethyl-3-{[2-({2-[(2-methylprop-2-enoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | CC(=C)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- N-glycosyl compound
- Glycosyl compound
- Beta amino acid or derivatives
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- N-acyl-amine
- Monosaccharide
- Fatty amide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Thiocarboxylic acid ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Thioether
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid amide
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Valine, leucine and isoleucine degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Lehnert W, Sass JO: Glutaconyl-CoA is the main toxic agent in glutaryl-CoA dehydrogenase deficiency (glutaric aciduria type I). Med Hypotheses. 2005;65(2):330-3. Pubmed: 15922108
- Shimomura, Y., Honda, T., Goto, H., Nonami, T., Kurokawa, T., Nagasaki, M., Murakami, T. (2004). "Effects of liver failure on the enzymes in the branched-chain amino acid catabolic pathway." Biochem Biophys Res Commun 313:381-385. Pubmed: 14684172
- Taniguchi K, Nonami T, Nakao A, Harada A, Kurokawa T, Sugiyama S, Fujitsuka N, Shimomura Y, Hutson SM, Harris RA, Takagi H: The valine catabolic pathway in human liver: effect of cirrhosis on enzyme activities. Hepatology. 1996 Dec;24(6):1395-8. Pubmed: 8938168
|
|---|
| Synthesis Reference: |
Hawes, John W.; Harper, Edwin T. Synthesis of methacrylyl-CoA and (R)- and (S)-3-hydroxyisobutyryl-CoA. Methods in Enzymology (2 |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|